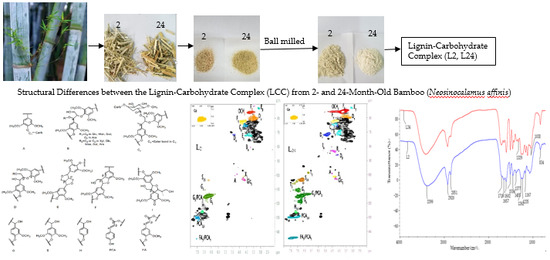
Structural Differences between the Lignin-Carbohydrate Complexes (LCCs) from 2- and 24-Month-Old Bamboo (Neosinocalamus affinis)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the Lignin-Carbohydrate Complex (LCC) Analysis
2.2. Fourier Transform Infrared (FT-IR) Spectra Analysis
2.3. HSQC NMR Spectra Analysis
2.3.1. Lignin Side Chain and Aromatic Regions
2.3.2. Major LCC Linkages
2.4. Molecular Weight Analysis
3. Materials and Methods
3.1. Preparation of Bamboo Culms Powder
3.2. Preparation of LCCs
3.3. Preparation of Hemicelluloses
3.4. Analytical Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Deutschmann, R.; Dekker, R.F. From plant biomass to bio-based chemicals: Latest developments in xylan research. Biotechnol. Adv. 2012, 30, 1627–1640. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Kawamura, I. Occurrence of p-hydroxyphenylglycerol-beta-aryl ether structure in lignins. Holzforschung 1966, 20, 16. [Google Scholar]
- Higuchi, T. Lignin biochemistry: Biosynthesis and biodegradation. Wood Sci. Technol. 1990, 24, 23–63. [Google Scholar] [CrossRef]
- Hu, Z.J.; Yeh, T.F.; Chang, H.M.; Matsumoto, Y.J.; Kadla, J.F. Elucidation of the structure of cellulolytic enzyme lignin. Holzforschung 2006, 50, 1040–1397. [Google Scholar] [CrossRef]
- Björkman, A. Lignin and lignin–carbohydrate complexes–extraction from wood meal with neutral solvents. Ind. Eng. Chem. 1957, 49, 1395–1398. [Google Scholar] [CrossRef]
- Toikka, M.; Sipilä, J.; Teleman, A.; Brunow, G. Lignin–carbohydrate model compounds. Formation of lignin–methyl arabinoside and lignin–methyl galactoside benzyl ethers via quinone methide intermediates. J. Chem. Soc. Perkin Trans. 1998, 22, 3813–3818. [Google Scholar] [CrossRef]
- Bjorkman, A. Studies on finely divided wood. Part III. Extraction of lignin-carbohydrate complexes with neutral solvents. Svensk Papperstidn 1957, 60, 243–251. [Google Scholar]
- Helm, R.F. Lignin—Polysaccharide Interactions in Woody Plants. In Lignin: Historical, Biolocal, and Merials Perspectives; Glasser, W.G., Northey, R.A., Schultz, T.P., Eds.; ACS Publications: Washington, DC, USA, 1999; Volume 742, pp. 161–171. [Google Scholar]
- Eriksson, Ö.; Goring, D.A.I.; Lindgren, B.O. Structural studies on the chemical bonds between lignins and carbohydrates in spruce wood. Wood Sci. Technol. 1980, 14, 267–279. [Google Scholar] [CrossRef]
- Imamura, T.; Watanabe, T.; Kuwahara, M.; Koshijima, T. Ester linkages between lignin and glucuronic acid in lignin-carbohydrate complexes from Fagus crenata. Phytochemistry 1994, 37, 1165–1173. [Google Scholar] [CrossRef]
- Karlsson, O.; Westermark, U. Evidence for chemical bonds between lignin and cellulose in kraft pulps. J. Pulp Pap. Sci. 1996, 22, J397–J401. [Google Scholar]
- Yaku, F.; Tsuji, S.; Koshijima, T. Lignin carbohydrate complex. Pt. III. Formation of micells in the aqueous solution of acidic lignin carbohydrate complex. Holzforschung 1979, 33, 54–59. [Google Scholar] [CrossRef]
- Aimi, H.; Matsumoto, Y.; Meshitsuka, G. Structure of small lignin fragments retained in water-soluble polysaccharides extracted from birch MWL isolation residue. J. Wood Sci. 2005, 51, 303–308. [Google Scholar] [CrossRef]
- Guerra, A.; Filpponen, I.; Lucia, L.A.; Argyropoulos, D.S. Comparative evaluation of three lignin isolation protocols for various wood species. J. Agric. Food Chem. 2006, 54, 9696–9705. [Google Scholar] [CrossRef] [PubMed]
- Azuma, J.I.; Takahashi, N.; Koshijima, T. Isolation and characterisation of lignin-carbohydrate complexes from the milled-wood lignin fraction of Pinus densiflora sieb. et zucc. Carbohydr. Res. 1981, 93, 91–104. [Google Scholar] [CrossRef]
- Freudenberg, K.; Grion, G. Beitrag zum Bildungsmechanismus des Lignins und der Lignin-Kohlenhydrat-Bindung. Eur. J. Inorg. Chem. 1959, 92, 1355–1363. [Google Scholar] [CrossRef]
- Zhang, B.; Fu, G.Q.; Niu, Y.S.; Peng, F.; Yao, C.L.; Sun, R.C. Variations of lignin–lignin and lignin–carbohydrate linkages from young Neosinocalamus affinis bamboo culms. RSC Adv. 2016, 6, 15478–15484. [Google Scholar] [CrossRef]
- Yin, H.S.; Liu, H.M.; Liu, Y.L. Structural Characterization of lignin in fruits and stalks of Chinese Quince. Molecules 2017, 22, 890. [Google Scholar] [CrossRef]
- Yoshida, S.; Kuno, A.; Saito, N.; Aoyama, M.; Kusakabe, I. Structure of xylan from culms of bamboo grass (Sasa senanensis Rehd.). J. Wood Sci. 1998, 44, 457–462. [Google Scholar] [CrossRef]
- Peng, P.; She, D. Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: A review. Carbohydr. Polym. 2014, 112, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Guan, Y.; Bian, J.; Peng, F.; Ren, J.L.; Yao, C.L.; Sun, R.C. Structure of hemicelluloses upon maturation of bamboo (Neosinocalamus affinis) culms. Cellul. Chem. Technol. 2016, 50, 189–198. [Google Scholar]
- Atsushi, K.; Azuma, J.; Koshijima, T. Lignin-carbohydrate complexes and phenolic acids in bagasse. Holzforschung 1984, 38, 141–149. [Google Scholar] [CrossRef]
- Bai, Y.Y.; Xiao, L.P.; Shi, Z.J.; Sun, R.C. Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; Xiao, L.P.; Meng, L.Y.; Zhang, X.M.; Sun, R.C. Isolation and structural characterization of lignin from cotton stalk treated in an ammonia hydrothermal system. Int. J. Mol. Sci. 2012, 13, 15209–15226. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.; Capanema, E.; Gracz, H.; Chang, H.M.; Jameel, H. Quantification of lignin–carbohydrate linkages with high-resolution NMR spectroscopy. Planta 2011, 233, 1097–1110. [Google Scholar] [CrossRef] [PubMed]
- Balakshin, M.Y.; Capanema, E.A.; Chang, H. MWL fraction with a high concentration of lignin-carbohydrate linkages: Isolation and 2D NMR spectroscopic analysis. Holzforschung 2007, 61, 1–7. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, Z.; Sun, Y.C.; Sun, S.N.; Xu, F.; Sun, R.C. Structural characterization of alkali-extractable lignin fractions from bamboo. J. Biobased Mater. Bioenergy 2010, 4, 408–425. [Google Scholar] [CrossRef]
- You, T.T.; Zhang, L.M.; Zhou, S.K.; Xu, F. Structural elucidation of lignin-carbohydrate complex (LCC) preparations and lignin from Arundo donax Linn. Ind. Crop. Prod. 2015, 71, 65–74. [Google Scholar] [CrossRef]
- Wen, J.L.; Xue, B.L.; Xu, F.; Sun, R.C.; Pinkert, A. Unmasking the structural features and property of lignin from bamboo. Ind. Crop. Prod. 2013, 42, 332–343. [Google Scholar] [CrossRef]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of lignin structures and lignin-carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 10604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Ibrahim, R.K. Tricin—A potential multifunctional nutraceutical. Phytochem. Rev. 2010, 9, 413–424. [Google Scholar] [CrossRef]
- Koshijima, T.; Watanabe, T. Association Between Lignin and Carbohydrates in Wood and Other Plant Tissues; Springer: Heidelberg, Gernmany, 2003; pp. 91–126. [Google Scholar]
- Adler, E. Lignin chemistry—Past, present and future. Wood Sci. Technol. 1977, 11, 169–218. [Google Scholar] [CrossRef]
- Terashima, N.; Ralph, S.A.; Landucci, L.L. New facile syntheses of monolignol glucosides; p-glucocoumaryl alcohol, coniferin and syringin. Holzforschung 1996, 50, 151–155. [Google Scholar]
- Zhang, L.; Gellerstedt, G. Quantitative 2D HSQC NMR determination of polymer structures by selecting suitable internal standard references. Magn. Reson. Chem. 2007, 45, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, K.; Harkin, J.M. Modelle für die Bindung des Lignins an die Kohlenhydrate. Eur. J. Inorg. Chem. 1960, 93, 2814–2819. [Google Scholar] [CrossRef]
- Tokimatsu, T.; Umezawa, T.; Shimada, M. Synthesis of four diastereomeric lignin carbohydrate complexes (LCC) model compounds composed of a β-O-4 lignin model linked to methyl β-d-glucoside. Holzforschung 1996, 50, 156–160. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report for Laboratory Analytical Procedure (LAP), NREL: Golden, CO, USA, 2008. [Google Scholar]
- Peng, F.; Ren, J.L.; Xu, F.; Bian, J.; Peng, P.; Sun, R.C. Fractionation of alkali-solubilized hemicelluloses from delignified Populus gansuensis: Structure and properties. J. Agric. Food Chem. 2010, 58, 5743–5750. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; Xu, F.; He, J.; Sun, R.C.; Liu, S.J.; Zhang, Z.S.; Scott, G. Structural and physico-chemical characterization of hemicelluloses from ultrasound-assisted extractions of partially delignified fast-growing poplar wood through organic solvent and alkaline solutions. Biotechnol. Adv. 2010, 28, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent Advances in Characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef] [PubMed]




| Sample | Yield a | Chemical Composition b (% of Relative Content) | Carbohydrate Content c (% of Relative Molar Content) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ASL | AIL | Carb | Ara | Gal | Glc | Xyl | Man | ||
| L2 | 3.9 | 51.9 ± 0.1 | 20.1 ± 0.3 | 28.0 ± 0.6 | 1.2 ± 0.1 | 5.0 ± 0.0 | 72.1 ± 0.3 | 18.7 ± 0.1 | 3.0 ± 0.0 |
| L24 | 1.5 | 10.9 ± 0.3 | 49.7 ± 0.7 | 39.4 ± 0.4 | 4.2 ± 0.1 | 2.2 ± 0.2 | 39.8 ± 0.2 | 52.2 ± 0.2 | 1.6 ± 0.1 |
| Sample a | Molar Composition b (Relative %, mol/mol) | Molar Ratio c | |||||
|---|---|---|---|---|---|---|---|
| Ara | Gal | Glc | Xyl | GlcA | GlcA/Xyl | Ara/Xyl | |
| H2 | 11.25 ± 0.2 | 4.60 ± 0.1 | 22.01 ± 0.1 | 58.30 ± 0.0 | 3.59 ± 0.1 | 0.06 | 0.19 |
| H24 | 6.48 ± 0.1 | 0.59 ± 0.0 | 2.87 ± 0.2 | 86.93 ± 0.0 | 3.30 ± 0.0 | 0.03 | 0.07 |
| Wave Numbers (cm−1) | Assignments |
|---|---|
| 3399 | O–H stretch |
| 2920 and 2851 | C–H stretch in methyl and methylene groups |
| 1719 | C=O stretch (unconjugated ketones, carbonyl and in ester groups in carbohydrate) |
| 1657 | Conjugated C=O stretch (lignin) |
| 1602 | Aromatic skeletal vibrations (lignin) |
| 1514 | Aromatic skeletal vibrations (lignin) |
| 1455 | Aromatic skeletal vibrations combined with C–H in-plane deform (lignin and methylene groups in polysaccharide) |
| 1377 | COO-asymmetric and symmetrical vibrations in carboxylate groups |
| 1329 | Syringyl units |
| 1262 | Guaiacyl units |
| 1235 | C–C, C–O, and C=O stretch of G ring |
| 1167 | Typical for HGS lignins; C=O in ester groups (conjugated) |
| 1038 | Aromatic C–H in-plane deformation, G > S; plus C–O deform, in primary alcohols; plus C=O stretch (unconjugated) |
| 834 | C–H out-of-plane in positions 2, 5, and 6 of G units |
| Label | δC/δH (ppm) | Assignments |
|---|---|---|
| -OCH3 | 56.09/3.72 | C–H in methoxyls |
| S2,6 | 104.74/6.67 | C2,6–H2,6 in syringyl units (S) |
| G2 | 111.82/6.97 | C2–H2 in guaiacyl units (G) |
| G5 | 115.89/6.76 | C5–H5 in guaiacyl units (G) |
| G6 | 119.34/6.76 | C6–H6 in guaiacyl units (G) |
| H2,6 | 128.22/7.19 | C2,6–H2,6 in p–hydroxyphenyl units (H) |
| Dα | 71.29/4.88 | Cα–Hα in β–O–4 structures linked to a S unit (D) |
| Dβ | 83.56/4.24 | Cβ–Hβ in β–O–4 structures linked to G/H units (D) |
| Dγ | 60.73/3.39 | Cγ–Hγ in β–O–4 structures (D) |
| Eβ | 52.97/3.09 | Cβ–Hβ in β–β structures (E) |
| Eγ | 70.26/3.96 | Cγ–Hγ in β–β structures (E) |
| Fβ | 53.57/3.39 | Cβ–Hβ in β–5 structures (F) |
| Fγ | 62.92/3.96 | Cγ–Hγ in β–5 structures (F) |
| Iα | 82.39/5.08 | Cα–Hα in β–1 structures (I) |
| Iβ | 59.62/2.72 | Cβ–Hβ in β–1 structures (I) |
| A | 100.5/4.88 | Phenyl glycoside linkages (A) |
| Bα | 82.5–80.0/4.7–4.3 | Cα–Hα in benzyl ether LCC bonds (B) |
| Cα | 77.0–75.0/6.2–6.0 | α–Ester (C) |
| Cγ | 65–62/4.5–4.0 | γ-Ester (C) |
| FA7/PCA7 | 144.76/7.43 | C7–H7 in p-coumaroylated substructures (PCA) and ferulate acid (FA) |
| PCA3 | 115.69/6.76 | C3–H3 in p-coumaroylated substructures (PCA) |
| PCA2,6 | 130.11/7.43 | C2,6–H2,6 in p-coumaroylated substructures (PCA) |
| X2 | 72.74/3.09 | C2–H2 in β–d–xylopyranoside (X) |
| X3 | 73.89/3.37 | C3–H3 in β–d–xylopyranoside (X) |
| X4 | 75.38/3.40 | C4–H4 in β–d–xylopyranoside (X) |
| GlcA2 | 72.74/3.37 | C2–H2 in glucuronic acid (GlcA) |
| GlcA3 | 73.89/3.48 | C3–H3 in glucuronic acid (GlcA) |
| X1 | 102.21/4.23 | C1–H1 in β–d–xylopyranoside (X) |
| Glc1 | 103.20/4.20 | C1–H1 in β–d–glucopyranoside (Glc) |
| T3 | 106.39/7.19 | C3–H3 in tricin |
| T6 | 99.82/6.22 | C2,6–H2,6 in tricin |
| Sample | LCC Linkages a | Average Molecular Weight (g/mol) | |||||
|---|---|---|---|---|---|---|---|
| Phenyl Glycoside | Benzyl Ether | α-Ester | S/G b | Mw | Mn | Mw/Mn | |
| L2 | 6.5 | 1.2 | 1.5 | 0.18 | 8650 | 8140 | 1.06 |
| L24 | 12.1 | 1.9 | 0.5 | 0.87 | 9890 | 9670 | 1.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, P.-P.; Hu, Y.-J.; Fu, G.-Q.; Sun, C.-X.; Li, M.-F.; Peng, F.; Sun, R.-C. Structural Differences between the Lignin-Carbohydrate Complexes (LCCs) from 2- and 24-Month-Old Bamboo (Neosinocalamus affinis). Int. J. Mol. Sci. 2018, 19, 1. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010001
Yue P-P, Hu Y-J, Fu G-Q, Sun C-X, Li M-F, Peng F, Sun R-C. Structural Differences between the Lignin-Carbohydrate Complexes (LCCs) from 2- and 24-Month-Old Bamboo (Neosinocalamus affinis). International Journal of Molecular Sciences. 2018; 19(1):1. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010001
Chicago/Turabian StyleYue, Pan-Pan, Ya-Jie Hu, Gen-Que Fu, Chang-Xia Sun, Ming-Fei Li, Feng Peng, and Run-Cang Sun. 2018. "Structural Differences between the Lignin-Carbohydrate Complexes (LCCs) from 2- and 24-Month-Old Bamboo (Neosinocalamus affinis)" International Journal of Molecular Sciences 19, no. 1: 1. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010001