Effects of Plant Oil Interesterified Triacylglycerols on Lipemia and Human Health
Abstract
:1. Introduction
2. Triacylglycerol (TAG) Structures and Chemical Properties in Plant Oil and Animal Fats
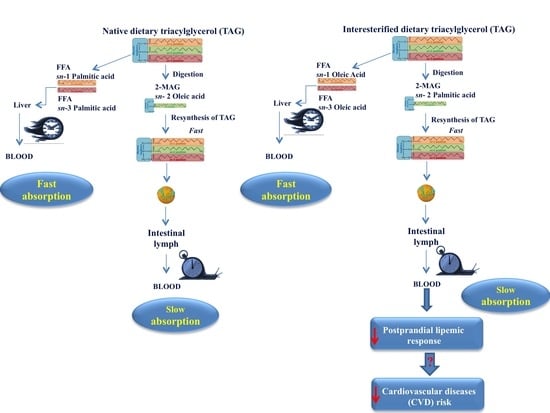
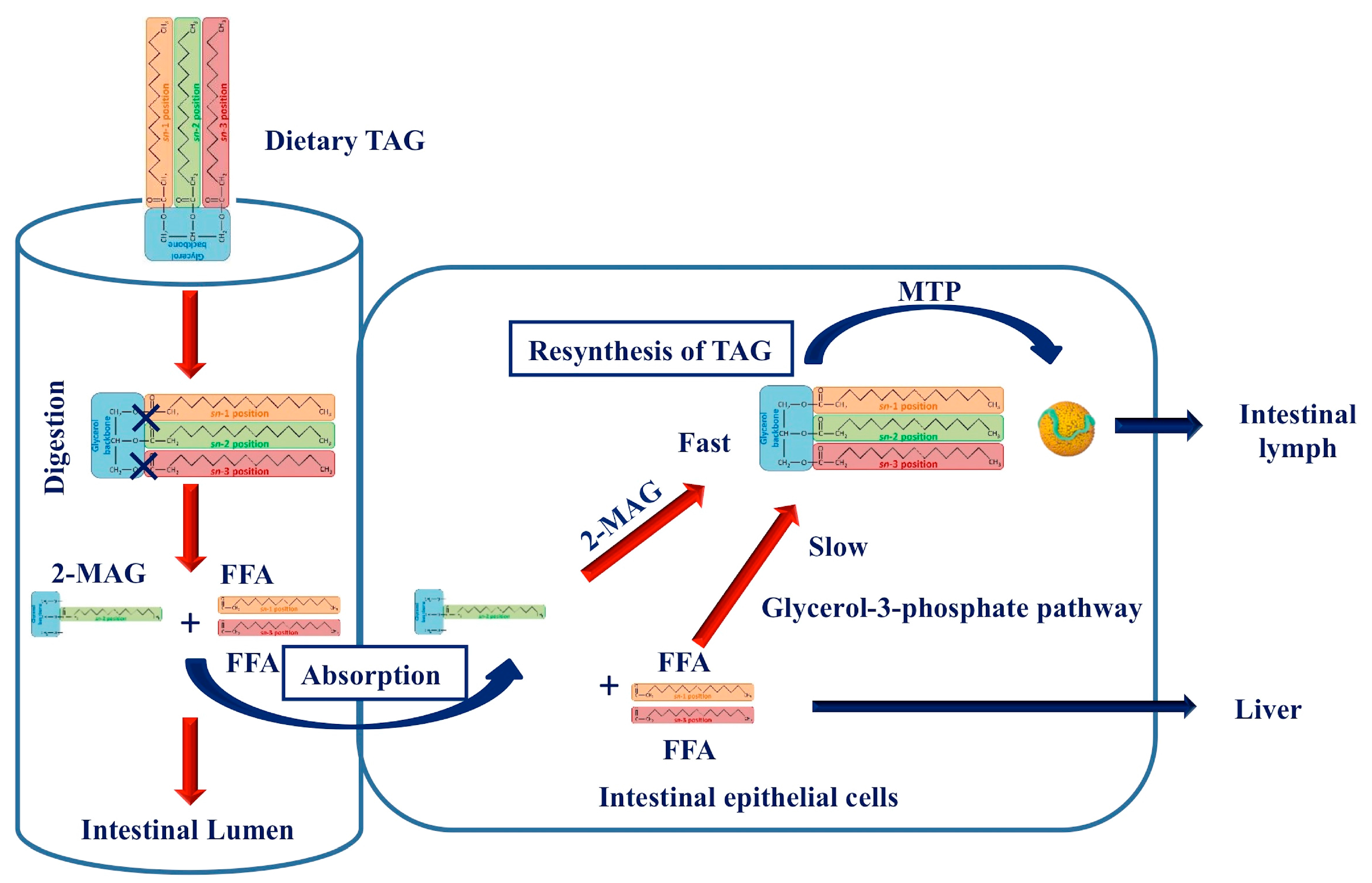
3. Postprandial Fate of Native and Interesterified Dietary Fats
4. Effect of Interesterified Fats on Fasting and Postprandial Lipemia
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2011, 155, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A. Lipid mediators in the neural cell nucleus: Their metabolism, signaling, and association with neurological disorders. Neuroscientist 2009, 15, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Strassner, C.; Cavoski, I.; di Cagno, R.; Kahl, J.; Kesse-Guyot, E.; Lairon, D.; Lampkin, N.; Løes, A.K.; Matt, D.; Niggli, U.; et al. How the Organic Food System Supports Sustainable Diets and Translates These into Practice. Front. Nutr. 2015, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Rioux, V.; Legrand, P. Saturated fatty acids: Simple molecular structures with complex cellular functions. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, M.G. The essentials of essential fatty acids. J. Diet. Suppl. 2009, 6, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Legrand, P.; Rioux, V. The complex and important cellular and metabolic functions of saturated fatty acids. Lipids 2010, 45, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Sundram, K.; Karupaiah, T.; Hayes, K.C. Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans. Nutr. Metab. 2007, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Lei, E.; Vacy, K.; Boon, W.C. Fatty acids and their therapeutic potential in neurological disorders. Neurochem. Int. 2016, 95, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wahrburg, U. What are the health effects of fat? Eur. J. Nutr. 2004, 43, i6–i11. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Martin, N.; Abdelhamid, A. Cochrane corner: What are the effects of reducing saturated fat intake on cardiovascular disease and mortality? Heart 2015, 101, 1938–1940. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [PubMed]
- Clifton, P.M.; Keogh, J.B. A systematic review of the effect of dietary saturated and polyunsaturated fat on heart disease. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1060–1080. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.P.; Voutilainen, S. Dietary fatty acids and risk of coronary heart disease in men: The Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2679–2687. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Chiu, S.; Bergeron, N.; Krauss, R.M. Saturated Fats versus Polyunsaturated Fats versus Carbohydrates for Cardiovascular Disease Prevention and Treatment. Annu. Rev. Nutr. 2015, 35, 517–543. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Mancini, A.; Imperlini, E.; Nigro, E.; Montagnese, C.; Daniele, A.; Orrù, S.; Buono, P. Biological and Nutritional Properties of Palm Oil and Palmitic Acid: Effects on Health. Molecules 2015, 20, 17339–17361. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Hu, F.B. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: An overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009, 22, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 2001, 36, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Hoy, C.E. The digestion of dietary triacylglycerols. Prog. Lipid Res. 2004, 43, 105–133. [Google Scholar] [CrossRef]
- Michalski, M.C.; Genot, C.; Gayet, C.; Lopez, C.; Fine, F.; Joffre, F.; Vendeuvre, J.L.; Bouvier, J.; Chardigny, J.M.; Raynal-Ljutovac, K.; et al. Multiscale structures of lipids in foods as parameters affecting fatty acid bioavailability and lipid metabolism. Prog. Lipid Res. 2013, 52, 354–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indelicato, S.; Bongiorno, D.; Pitonzo, R.; Di Stefano, V.; Calabrese, V.; Indelicato, S.; Avellone, G. Triacylglycerols in edible oils: Determination, characterization, quantitation, chemometric approach and evaluation of adulterations. J. Chromatogr. A 2017, 1515, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, M.; Scolamiero, E.; Caterino, M.; Mirisola, V.; Franconi, F.; Campesi, I. Female and male human babies have distinct blood metabolomic patterns. Mol. Biosyst. 2015, 11, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Ruoppolo, M.; Campesi, I.; Scolamiero, E.; Pecce, R.; Caterino, M.; Cherchi, S.; Mercuro, G.; Tonolo, G.; Franconi, F. Serum metabolomic profiles suggest influence of sex and oral contraceptive use. Am. J. Transl. Res. 2014, 6, 614–624. [Google Scholar] [PubMed]
- Karupaiah, T.; Sundram, K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: A review of their nutritional implications. Nutr. Metab. 2007, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, X. Effects of Lipid Structure Changed by Interesterification on Melting Property and Lipemia. Lipids 2016, 51, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Schulthess, G.; Lipka, G.; Compassi, S.; Boffelli, D.; Weber, F.E.; Paltauf, F.; Hauser, H. Absorption of monoacylglycerols by small intestinal brush border membrane. Biochemistry 1994, 33, 4500–4508. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Kuksis, A. Apparent convergence (at 2-monoacylglycerol level) of phosphatidic acid and 2-monoacylglycerol pathways of synthesis of chylomicron triacylglycerols. J. Lipid Res. 1991, 32, 1173–1186. [Google Scholar] [PubMed]
- Emken, E.A.; Adlof, R.O.; Duval, S.M.; Shane, J.M.; Walker, P.M.; Becker, C. Effect of triacylglycerol structure on absorption and metabolism of isotope-labeled palmitic and linoleic acids by humans. Lipids 2004, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Sanders, T.A.; Baer, D.J.; Hayes, K.C.; Howles, P.N.; Marangoni, A. The Increasing Use of Interesterified Lipids in the Food Supply and Their Effects on Health Parameters. Adv. Nutr. 2016, 7, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Porsgaard, T.; Hoy, C.E. Lymphatic transport in rats of several dietary fats differing in fatty acid profile and triacylglycerol structure. J. Nutr. 2000, 130, 1619–1624. [Google Scholar] [PubMed]
- Nagata, J.; Kasai, M.; Watanabe, S.; Ikeda, I.; Saito, M. Effects of highly purified structured lipids containing medium-chain fatty acids and linoleic acid on lipid profiles in rats. Biosci. Biotechnol. Biochem. 2003, 67, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.Q.; Huang, J.H.; Jin, Q.Z.; Liu, Y.F.; Song, Z.H.; Wang, X.G. Lipase-catalyzed preparation of human milk fat substitutes from palm stearin in a solvent-free system. J. Agric. Food Chem. 2011, 59, 6055–6063. [Google Scholar] [CrossRef] [PubMed]
- Dollah, S.; Abdulkarim, S.M.; Ahmad, S.H.; Khoramnia, A.; Mohd Ghazali, H. Physico-chemical properties of Moringa oleifera seed oil enzymatically interesterified with palm stearin and palm kernel oil and its potential application in food. J. Sci. Food Agric. 2016, 96, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Farfan, M.; Villalon, M.J.; Ortiz, M.E.; Nieto, S.; Bouchon, P. The effect of interesterification on the bioavailability of fatty acids in structured lipids. Food Chem. 2013, 139, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rogers, M.; Lan, Y.; Huynh, S.; Marangoni, A.G.; Robinson, L.E.; Wright, A.J. Investigations of in vitro bioaccessibility from interesterified stearic and oleic acid-rich blends. Food Funct. 2016, 7, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Kallio, H.; Nylund, M.; Bostrom, P.; Yang, B. Triacylglycerol regioisomers in human milk resolved with an algorithmic novel electrospray ionization tandem mass spectrometry method. Food Chem. 2017, 233, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.; Amate, L.; Gil, A. Absorption and distribution of dietary fatty acids from different sources. Early Hum. Dev. 2001, 65, S95–S101. [Google Scholar] [CrossRef]
- Lucas, A.; Quinlan, P.; Abrams, S.; Ryan, S.; Meah, S.; Lucas, P.J. Randomised controlled trial of a synthetic triglyceride milk formula for preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 77, F178–F184. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.J.; Howes, D.; Earl, L.K. The absorption, distribution and excretion of 1- and 2-. Food Chem. Toxicol. 2001, 39, 709–716. [Google Scholar] [CrossRef]
- Miles, E.A.; Calder, P.C. The influence of the position of palmitate in infant formula triacylglycerols on health outcomes. Nutr. Res. 2017, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stender, S.; Astrup, A.; Dyerberg, J. Tracing artificial trans fat in popular foods in Europe: A market basket investigation. BMJ Open 2014, 4, e005218. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Katan, M.B.; Ascherio, A.; Stampfer, M.J.; Willett, W.C. Trans fatty acids and cardiovascular disease. N. Engl. J. Med. 2006, 354, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Hall, W.L.; Berry, S.E.E. What are interesterified fats and should we be worried about them in our diet? Nutr. Bull. 2017, 42, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Teeman, C.S.; Kurti, S.P.; Cull, B.J.; Emerson, S.R.; Haub, M.D.; Rosenkranz, S.K. Postprandial lipemic and inflammatory responses to high-fat meals: A review of the roles of acute and chronic exercise. Nutr. Metab. 2016, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Rifai, N.; Buring, J.E.; Ridker, P.M. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation 2008, 118, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- De Backer, G.; Ambrosioni, E.; Borch-Johnsen, K.; Brotons, C.; Cifkova, R.; Dallongeville, J.; Ebrahim, S.; Faergeman, O.; Graham, I.; Mancia, G.; et al. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis 2003, 171, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Mikhailidis, D.P.; Nordestgaard, B.G.; Bilianou, H.; Panotopoulos, G. Definition of postprandial lipaemia. Curr. Vasc. Pharmacol. 2011, 9, 292–301. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Gheewala, N.M.; O’Keefe, J.O. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J. Am. Coll. Cardiol. 2008, 51, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Tholstrup, T.; Miller, G.J.; Bysted, A.; Sandstrom, B. Effect of individual dietary fatty acids on postprandial activation of blood coagulation factor VII and fibrinolysis in healthy young men. Am. J. Clin. Nutr. 2003, 77, 1125–1132. [Google Scholar] [PubMed]
- Renaud, S.C.; Ruf, J.C.; Petithory, D. The positional distribution of fatty acids in palm oil and lard influences their biologic effects in rats. J. Nutr. 1995, 125, 229–237. [Google Scholar] [PubMed]
- Kritchevsky, D. Trans fatty acid effects in experimental atherosclerosis. Fed. Proc. 1982, 41, 2813–2817. [Google Scholar] [PubMed]
- Redgrave, T.G.; Kodali, D.R.; Small, D.M. The effect of triacyl-sn-glycerol structure on the metabolism of chylomicrons and triacylglycerol-rich emulsions in the rat. J. Biol. Chem. 1988, 263, 5118–5123. [Google Scholar] [PubMed]
- Nelson, C.M.; Innis, S.M. Plasma lipoprotein fatty acids are altered by the positional distribution of fatty acids in infant formula triacylglycerols and human milk. Am. J. Clin. Nutr. 1999, 70, 62–69. [Google Scholar] [PubMed]
- Berry, S.E.; Woodward, R.; Yeoh, C.; Miller, G.J.; Sanders, T.A. Effect of interesterification of palmitic acid-rich triacylglycerol on postprandial lipid and factor VII response. Lipids 2007, 42, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Christophe, A.B.; de Greyt, W.F.; Delanghe, J.R.; Huyghebaert, A.D. Substituting enzymatically interesterified butter for native butter has no effect on lipemia or lipoproteinemia in Man. Ann. Nutr. Metab. 2000, 44, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Filippou, A.; Teng, K.T.; Berry, S.E.; Sanders, T.A. Palmitic acid in the sn-2 position of dietary triacylglycerols does not affect insulin secretion or glucose homeostasis in healthy men and women. Eur. J. Clin. Nutr. 2014, 68, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Zock, P.L.; de Vries, J.H.; de Fouw, N.J.; Katan, M.B. Positional distribution of fatty acids in dietary triglycerides: effects on fasting blood lipoprotein concentrations in humans. Am. J. Clin. Nutr. 1995, 61, 48–55. [Google Scholar] [PubMed]
- Sanders, T.A.; Filippou, A.; Berry, S.E.; Baumgartner, S.; Mensink, R.P. Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am. J. Clin. Nutr. 2011, 94, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Brito, M.F.; Huang, J.; Wood, L.V.; Filippou, A.; Sanders, T.A.; Berry, S.E. An interesterified palm olein test meal decreases early-phase postprandial lipemia compared to palm olein: a randomized controlled trial. Lipids 2014, 49, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, W.L.; Iqbal, S.; Li, H.; Gray, R.; Berry, S.E.E. Modulation of postprandial lipaemia by a single meal containing a commonly consumed interesterified palmitic acid-rich fat blend compared to a non-interesterified equivalent. Eur. J. Nutr. 2017, 56, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Berry, S.E.; Miller, G.J. Influence of triacylglycerol structure on the postprandial response of factor VII to stearic acid-rich fats. Am. J. Clin. Nutr. 2003, 77, 777–782. [Google Scholar] [PubMed]
- Berry, S.E.; Miller, G.J.; Sanders, T.A. The solid fat content of stearic acid-rich fats determines their postprandial effects. Am. J. Clin. Nutr. 2007, 85, 1486–1494. [Google Scholar] [PubMed]
- Afonso, M.S.; Lavrador, M.S.; Koike, M.K.; Cintra, D.E.; Ferreira, F.D.; Nunes, V.S.; Castilho, G.; Gioielli, L.A.; Paula Bombo, R.; Catanozi, S.; et al. Dietary interesterified fat enriched with palmitic acid induces atherosclerosis by impairing macrophage cholesterol efflux and eliciting inflammation. J. Nutr. Biochem. 2016, 32, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Jackson, K.G.; Poppitt, S.D.; Minihane, A.M. Postprandial lipemia and cardiovascular disease risk: Interrelationships between dietary, physiological and genetic determinants. Atherosclerosis 2012, 220, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58, 25145. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Plant Oil | TAG | Animal Fat | TAG | ||||
|---|---|---|---|---|---|---|---|
| Type | sn-1 | sn-2 | sn-3 | Type | sn-1 | sn-2 | sn-3 |
| Cocoa butter | P | O | P | Milk (cow) | P | P | B |
| P | O | S | O | P | B | ||
| S | O | S | P | M | B | ||
| Palm oil | P | O | P | Lard (pig) | S | P | O |
| P | O | L | O | P | L | ||
| P | O | O | O | P | O | ||
| Soybean oil | L | L | P | Tallow (beef) | P | O | P |
| L | L | L | P | S | O | ||
| L | L | O | P | O | O | ||
| Peanut oil | P | O | L | Butter | P | P | B |
| O | L | L | P | P | C | ||
| O | O | L | P | O | P | ||
| Olive oil | O | O | P | Horse fat | P | O | O |
| O | O | O | O | O | O | ||
| O | L | O | L | O | O | ||
| Coconut oil | D | D | D | Egg | P | O | O |
| C | D | D | P | L | O | ||
| C | D | M | P | O | S | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, A.; Imperlini, E.; Nigro, E.; Vitucci, D.; Orrù, S.; Daniele, A.; Buono, P.; Mancini, A. Effects of Plant Oil Interesterified Triacylglycerols on Lipemia and Human Health. Int. J. Mol. Sci. 2018, 19, 104. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010104
Alfieri A, Imperlini E, Nigro E, Vitucci D, Orrù S, Daniele A, Buono P, Mancini A. Effects of Plant Oil Interesterified Triacylglycerols on Lipemia and Human Health. International Journal of Molecular Sciences. 2018; 19(1):104. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010104
Chicago/Turabian StyleAlfieri, Andreina, Esther Imperlini, Ersilia Nigro, Daniela Vitucci, Stefania Orrù, Aurora Daniele, Pasqualina Buono, and Annamaria Mancini. 2018. "Effects of Plant Oil Interesterified Triacylglycerols on Lipemia and Human Health" International Journal of Molecular Sciences 19, no. 1: 104. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010104