The Osteogenic Differentiation Effect of the FN Type 10-Peptide Amphiphile on PCL Fiber
Abstract
:1. Introduction
2. Results and Discussion
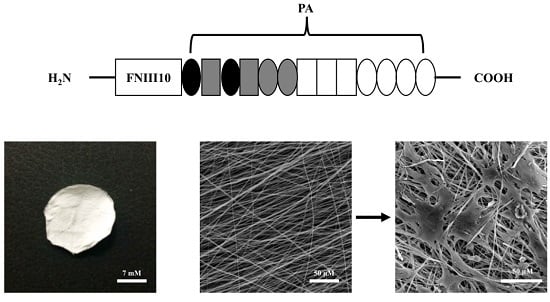
2.1. Morphology of PCL Fibers
2.2. Protein Adhesion Activity of FNIII10-PA on PCL Fibers
2.3. Cell Adhesion Activity of FNIII10-PA on PCL Fibers
2.4. Cell Proliferation Activity of FNIII10-PA on PCL Fiber
2.5. The Osteogenic Differentiation Activity of FNIII10-PA on PCL Fibers
3. Materials and Methods
3.1. Protein Expression and Purification
3.2. Electrospinning of PCL Fibers
3.3. Morphology of PCL Fibers and Cells on PCL Fibers
3.4. Protein Adhesion Assay
3.5. Cell Culture
3.6. Cell Adhesion Assay
3.7. Cell Proliferation Assay
3.8. Quantitative Real-Time PCR Analysis
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shin, H.; Jo, S.; Mikos, A.G. Biomimetic materials for tissue engineering. Biomaterials 2003, 24, 4353–4364. [Google Scholar] [CrossRef]
- Goktas, M.; Cinar, G.; Orujalipoor, I.; Ide, S.; Tekinay, A.B.; Guler, M.O. Self-assembled peptide amphiphile nanofibers and peg composite hydrogels as tunable ECM mimetic microenvironment. Biomacromolecules 2015, 16, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Patterson, J.L.; Vines, J.B.; Javed, A.; Gilbert, S.R.; Jun, H.W. Biphasic peptide amphiphile nanomatrix embedded with hydroxyapatite nanoparticles for stimulated osteoinductive response. ACS Nano 2011, 5, 9463–9479. [Google Scholar] [CrossRef] [PubMed]
- Shroff, K.; Pearce, T.R.; Kokkoli, E. Enhanced integrin mediated signaling and cell cycle progression on fibronectin mimetic peptide amphiphile monolayers. Langmuir 2012, 28, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, M.; Anderson, J.M.; Bosworth, C.A.; Andukuri, A.; Minor, W.P.; Lancaster, J.R., Jr.; Anderson, P.G.; Brott, B.C.; Jun, H.W. A nitric oxide releasing, self-assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials 2010, 31, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, B.; Meyer, J.; Jonczyk, A.; Kessler, H.; Adamietz, P.; Meenen, N.M.; Kantlehner, M.; Goepfert, C.; Nies, B. RGD-peptides for tissue engineering of articular cartilage. Biomaterials 2002, 23, 3455–3463. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, L.; Wang, X.; Zhang, X.; Liu, M.; Zeng, W. Integrin (αvβ3) tageted RGD peptide based probe for cancer optical imaging. Curr. Protein Pept. Sci. 2016, 17, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Diao, Y.; Li, W.; Yang, Z.; Zhang, L.; Chen, Z.; Wu, Y. RGD peptide conjugation results in enhanced antitumor activity of PD0325901 against glioblastoma byboth tumor-targeting delivery and combination therapy. Int. J. Pharm. 2016, 505, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lin, Y.; Zhang, X.; Feng, C.; Lu, Y.; Gao, Y.; Dong, C. Cyclic RGD peptide-modified liposomal drug delivery system for targeted oral apatinib administration: Enhanced cellular uptake and improved therapeutic effects. Int. J. Nanomed. 2017, 12, 1941–1958. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhou, H.; Weir, M.D.; Tang, M.; Bao, C.; Xu, H.H. Human embryonic stem cell-derived mesenchymal stem cell seeding on calcium phosphate cement-chitosan-RGD scaffold for bone repair. Tissue Eng. Part A 2013, 19, 915–927. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, B.; Xiao, X.; Meng, Q.; Li, W.; Yu, Q.; Bi, J.; Cheng, Y.; Qu, Z. Preparation and evaluation of an Arg-Gly-Asp-modified chitosan/hydroxyapatite scaffold for application in bone tissue engineering. Mol. Med. Rep. 2015, 12, 7263–7270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D.; Heo, J.; Hwang, Y.; Kwak, S.Y.; Park, O.K.; Kim, H.; Varghese, S.; Hwang, N.S. Extracellular-matrix-based and Arg-Gly-Asp-modified photopolymerizing hydrogels for cartilage tissue engineering. Tissue Eng. Part A 2015, 21, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, S.; Çakmak, A.S.; Gümüşderelioğlu, M. RGD-bearing peptide-amphiphile-hydroxyapatite nanocomposite bone scaffold: An in vitro study. Biomed. Mater. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Pham, L.B.H.; Yoo, Y.R.; Lee, S.; Kim, H.W.; Jang, J.H. Engineering of self-assembled fibronectin matrix protein and its effects on mesenchymal stem cells. Int. J. Mol. Sci. 2015, 16, 19645–19656. [Google Scholar] [CrossRef] [PubMed]
- Berns, E.J.; Álvarez, Z.; Goldberger, J.E.; Boekhoven, J.; Kessler, J.A.; Kuhn, H.G.; Stupp, S.I. A tenascin-C mimetic peptide amphiphile nanofiber gel promotes neurite outgrowth and cell migration of neurosphere-derived cells. Acta Biomater. 2016, 37, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Lizárraga, K.K.; Flores-Morales, C.; Del Prado-Audelo, M.L.; Álvarez-Pérez, M.A.; Piña-Barba, M.C.; Escobedo, C. Polycaprolactone- and polycaprolactone/ceramic-based 3D-bioplotted porous scaffolds for bone regeneration: A comparative study. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Miszuk, J.M.; Zhao, Y.; Sun, H.; Fong, H. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv. Healthc. Mater. 2015, 4, 2238–2246. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 620–634. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, G. Electrospun PCL/phlorotannin nanofibres for tissue engineering: Physical properties and cellular activities. Carbohydr. Polym. 2012, 90, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Chang, Y.S. Preparation and characterization of composite nanofibers of polycaprolactone and nanohydroxyapatite for osteogenic differentiation of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2011, 86, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Mohamadyar-Toupkanlou, F.; Vasheghani-Farahani, E.; Hanaee-Ahvaz, H.; Soleimani, M.; Dodel, M.; Havasi, P.; Ardeshirylajimi, A.; Taherzadeh, E.S. Osteogenic differentiation of MSCs on fibronectin-coated and nHA-modified scaffolds. ASAIO J. 2017, 63, 684–691. [Google Scholar] [CrossRef] [PubMed]
- Shekaran, A.; Lam, A.; Sim, E.; Jialing, L.; Jian, L.; Wen, J.T.; Chan, J.K.; Choolani, M.; Reuveny, S.; Birch, W.; et al. Biodegradable ECM-coated PCL microcarriers support scalable human early MSC expansion and in vivo bone formation. Cytotherapy 2016, 18, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.H.; Abroun, S.; Soleimani, M.; Mowla, S.J. Expansion of human cord blood hematopoietic stem/progenitor cells in three-dimensional Nanoscaffold coated with Fibronectin. Int. J. Hematol. Oncol. Stem Cell Res. 2015, 9, 72–79. [Google Scholar] [PubMed]
- Drevelle, O.; Bergeron, E.; Senta, H.; Lauzon, M.A.; Roux, S.; Grenier, G.; Faucheux, N. Effect of functionalized polycaprolactone on the behaviour of murine preosteoblasts. Biomaterials 2010, 31, 6468–6476. [Google Scholar] [CrossRef] [PubMed]
- Won, J.E.; Mateos-Timoneda, M.A.; Castano, O.; Planell, J.A.; Seo, S.J.; Lee, E.J.; Han, C.M.; Kim, H.W. Fibronectin immobilization on to robotic-dispensed nanobioactive glass/polycaprolactone scaffolds for bone tissue engineering. Biotechnol. Lett. 2015, 37, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.P.; Kim, S.J.; Lim, Y.M.; Park, K.; Kim, H.J.; Jeong, S.I.; Kim, S.E.; Song, H.R. The effect of alendronate-loaded polycarprolactone nanofibrous scaffolds on osteogenic differentiation of adipose-derived stem cells in bone tissue regeneration. J. Biomed. Nanotechnol. 2014, 10, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Marom, R.; Shur, I.; Solomon, R.; Benayahu, D. Characterization of adhesion and differentiation markers of osteogenic marrow stromal cells. J. Cell. Physiol. 2005, 202, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.T.; Li, W.J.; Tuan, R.S.; Chang, W.H. Modulation of osteogenesis in human mesenchymal stem cells by specific pulsed electromagnetic field stimulation. J. Orthop. Res. 2009, 27, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Zohar, R.; Cheifetz, S.; McCulloch, C.A.; Sodek, J. Analysis of intracellular osteopontin as a marker of osteoblastic cell differentiation and mesenchymal cell migration. Eur. J. Oral Sci. 1998, 106 (Suppl. S1), 401–407. [Google Scholar] [CrossRef] [PubMed]
- Candeliere, G.A.; Liu, F.; Aubin, J.E. Individual osteoblasts in the developing calvaria express different gene repertoires. Bone 2001, 28, 351–361. [Google Scholar] [CrossRef]
- Li, S.; Kong, H.; Yao, N.; Yu, Q.; Wang, P.; Lin, Y.; Wang, J.; Kuang, R.; Zhao, X.; Xu, J.; et al. The role of runt-related transcription factor 2 (Runx2) in the late stage of odontoblast differentiation and dentin formation. Biochem. Biophys. Res. Commun. 2011, 410, 698–704. [Google Scholar] [CrossRef] [PubMed]





| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | TCCACTCACGGCAAATTCAAC | AGCCCAAGATGCCCTTCAGT |
| ALP | GGGCAATGAGGTCACATCC | GTCACAATGCCCACGGACTT |
| BSP | CAGAGGAGGCAAGCGTCACT | CTGTCTGGGTGCCAACACTG |
| Col I | GAGGCATAAAGGGTATCGTGG | CATTAGGCGCAGGAAGGTCAGC |
| OC | CATCACTGCCACCCAGAAGAC | CAGTGGATGCAGGGATGATGT |
| OPN | CCAATGAAAGCCATGACCAC | CGACTGTAGGGACGATTGGA |
| Runx2 | GGCCGGGAATGATGAGAACTA | GGCCCACAAATCTCAGATCGT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, Y.-R.; Kim, H.-W.; Jang, J.-H. The Osteogenic Differentiation Effect of the FN Type 10-Peptide Amphiphile on PCL Fiber. Int. J. Mol. Sci. 2018, 19, 153. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010153
Yun Y-R, Kim H-W, Jang J-H. The Osteogenic Differentiation Effect of the FN Type 10-Peptide Amphiphile on PCL Fiber. International Journal of Molecular Sciences. 2018; 19(1):153. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010153
Chicago/Turabian StyleYun, Ye-Rang, Hae-Won Kim, and Jun-Hyeog Jang. 2018. "The Osteogenic Differentiation Effect of the FN Type 10-Peptide Amphiphile on PCL Fiber" International Journal of Molecular Sciences 19, no. 1: 153. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010153