Mapping the Schizophrenia Genes by Neuroimaging: The Opportunities and the Challenges
Abstract
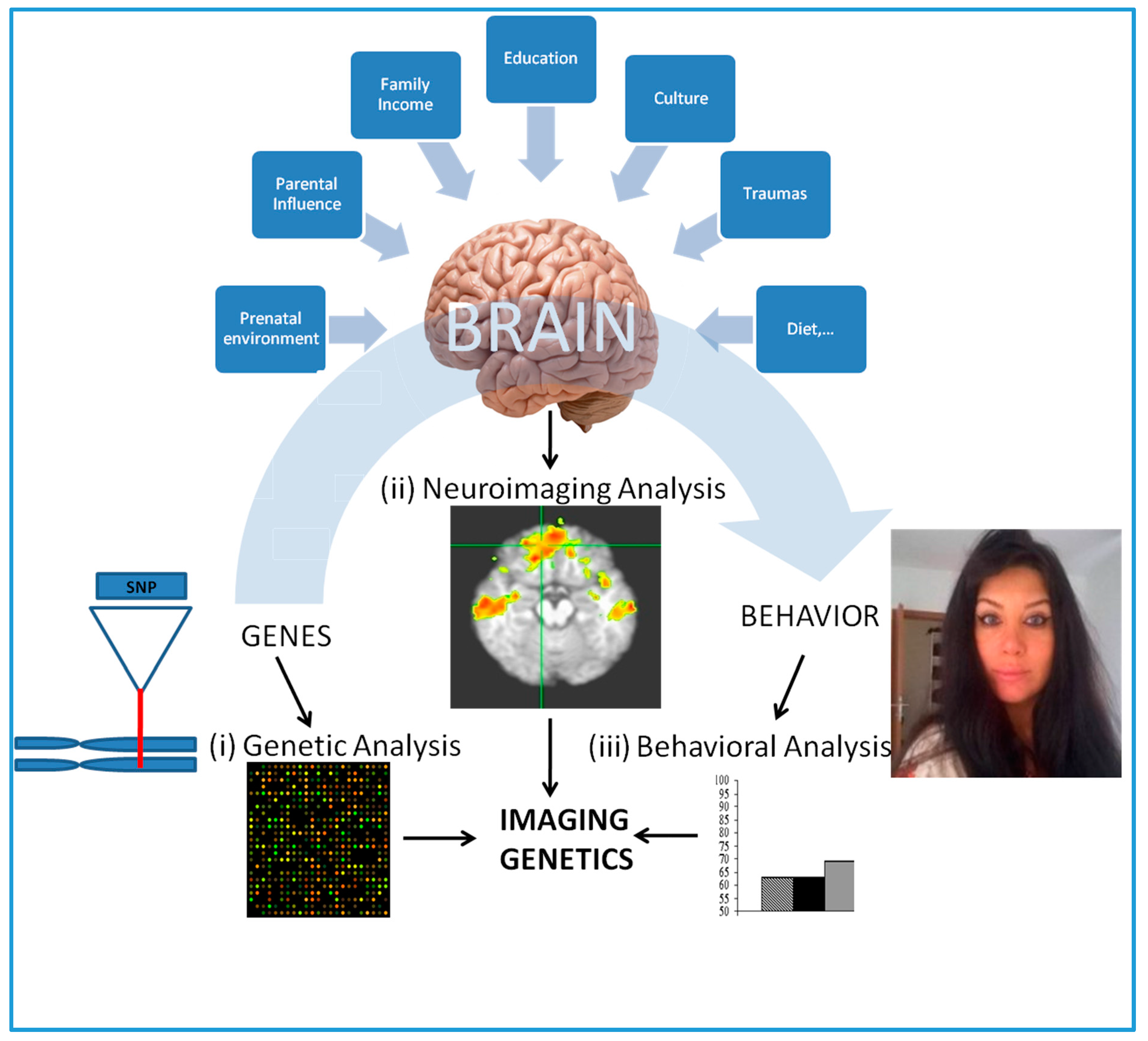
:1. Introduction
1.1. Imaging Genetics
1.2. A Controversial SZ Candidate Gene Encoding for Catechol-O-methyltransferase (COMT) and Others
2. The New Age
2.1. Novel SZ Candidate Genes: The Cherry on the Cake
2.2. Large Scale Multivariate Data: The Low Hanging Fruits
3. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ADHD | Attention deficit hyperactivity disorder |
| BDNF | Brain derived neurotrophic factor |
| CACNA1C | Calcium voltage-gated channel subunit α1 C-gene |
| CNV | Copy number variation |
| COMT | Catechol-O-methyltransferase |
| DLPFC | Dorsolateral prefrontal cortex |
| fMRI | Functional magnetic resonance imaging |
| GWAS | Genome wide association studies |
| IG | Imaging genetics |
| miRNAs | Micro RNA |
| MHC | Major histocompatibility complex |
| MRI | Magnetic resonance imaging |
| PGC | Psychiatric genetics consortium |
| SZ | Schizophrenia |
References
- Pollard, K.S.; Salama, S.R.; Lambert, N.; Lambot, M.A.; Coppens, S.; Pedersen, J.S.; Katzman, S.; King, B.; Onodera, C.; Siepel, A.; et al. An RNA gene expressed during cortical development evolved rapidly in humans. Nature 2006, 443, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.S.; Salama, S.R.; King, B.; Kern, A.D.; Dreszer, T.; Katzman, S.; Siepel, A.; Pedersen, J.S.; Bejerano, G.; Baertsch, R.; et al. Forces shaping the fastest evolving regions in the human genome. PLoS Genet. 2006, 2, e168. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Schadt, E.E.; Pollard, K.S.; Roussos, P.; Dudley, J.T. Genomic and network patterns of schizophrenia genetic variation in human evolutionary accelerated regions. Mol. Biol. Evol. 2015, 32, 1148–1160. [Google Scholar] [CrossRef] [PubMed]
- Götz, T.; Arslan, A.; Wisden, W.; Wulff, P. GABA (A) receptors: Structure and function in the basal ganglia. Prog. Brain Res. 2007, 160, 21–41. [Google Scholar]
- Schork, A.J.; Wang, Y.; Thompson, W.K.; Dale, A.M.; Andreassen, O.A. New statistical approaches exploit the polygenic architecture of schizophrenia—Implications for the underlying neurobiology. Curr. Opin. Neurobiol. 2016, 36, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A. Genes, brains, and behavior: Imaging genetics for neuropsychiatric disorders. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, I.I.; Gould, T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiatry 2003, 160, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Rose, E.J.; Donohoe, G. Brain vs. behavior: An effect size comparison of neuroimaging and cognitive studies of genetic risk for schizophrenia. Schizophr. Bull. 2013, 39, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Mier, D.; Kirsch, P.; Meyer-Lindenberg, A. Neural substrates of pleiotropic action of genetic variation in COMT: A meta-analysis. Mol. Psychiatry 2010, 15, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Weinberger, D.R. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat. Rev. Neurosci. 2006, 7, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A. The future of fMRI and genetics research. Neuroimage 2012, 15, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Tost, H.; Bilek, E.; Meyer-Lindenberg, A. Brain connectivity in psychiatric imaging genetics. Neuroimage 2012, 1, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, R.; Salmeron, B.J.; Carey, C.E.; Agrawal, A.; Calhoun, V.D.; Garavan, H.; Hariri, A.R.; Heinz, A.; Hill, M.N.; Holmes, A. Imaging genetics and genomics in psychiatry: A critical review of progress and potential. Biol. Psychiatry 2017, 82, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.S.; Arias Vásquez, A.; Franke, B.; Hoekstra, P.J.; Heslenfeld, D.J.; Oosterlaan, J.; Faraone, S.V.; Buitelaar, J.K.; Hartman, C.A. Developmentally sensitive interaction effects of genes and the social environment on total and subcortical brain volumes. PLoS ONE 2016, 11, e0155755. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A. Imaging genetics of schizophrenia in the post-GWAS era. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.J.; Hywel, J.; Williams, B.G.; Williams, N.M.; Norton, N.; Zammit, S.; Macgregor, S.; Kirov, G.K.; Owen, M.J.; O’Donovan, M.C. No association between schizophrenia and polymorphisms in COMT in two large samples. Am. J. Psychiatry 2005, 162, 1736–1738. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.S.; Werge, T.; Sklar, P.; Owen, M.J.; Ophoff, R.A.; O’Donovan, M.C.; Corvin, A.; Cichon, S.; Sullivan, P.F. Evaluating historical candidate genes for schizophrenia. Mol. Psychiatry 2015, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Risch, N. Linkage strategies for genetically complex traits. I. multilocus models. Am. J. Hum. Genet. 1990, 46, 222–228. [Google Scholar] [PubMed]
- Risch, N.; Merikangas, K. The future of genetic studies of complex human diseases. Science 1996, 273, 1516–1517. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, X.; O’Neill, A.F.; Walsh, D.; Kendler, K.S. Variants in the catechol-O-methyltransferase (COMT) gene are associated with schizophrenia in Irish high-density families. Mol. Psychiatry 2004, 9, 962–967. [Google Scholar] [CrossRef] [PubMed]
- Shifman, S.; Bronstein, M.; Sternfeld, M.; Pisante, A.; Weizman, A.; Reznik, I.; Spivak, B.; Grisaru, N.; Karp, L.; Schiffer, R. COMT: A common susceptibility gene in bipolar disorder and schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004, 128B, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Sirotin, Y.B.; Das, A. Anticipatory haemodynamic signals in sensory cortex not predicted by local neuronal activity. Nature 2009, 22, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Vul, E.; Harris, C.; Winkielman, P.; Pashler, H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect. Psychol. Sci. 2009, 4, 274–290. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.M.; Baird, A.A.; Miller, M.B.; Wolford, G.L. Neural correlates of interspecies perspective taking in the post-mortem Atlantic salmon: An argument for proper multiple comparisons correction. J. Serendipitous Unexpect. Results 2011, 1, 1–5. [Google Scholar] [CrossRef]
- Uğurbil, K. Imaging at ultrahigh magnetic fields: History, challenges, and solutions. Neuroimage 2017. [Google Scholar] [CrossRef] [PubMed]
- Knickmeyer, R.C.; Wang, J.; Zhu, H.; Geng, X.; Woolson, S.; Hamer, R.M.; Konneker, T.; Lin, W.; Styner, M.; Gilmore, J.H. Common variants in psychiatric risk genes predict brain structure at birth. Cereb. Cortex 2014, 24, 1230–1246. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, D.P.; Ianni, A.M.; Wei, S.M.; Kohn, P.D.; Kolachana, B.; Apud, J.; Weinberger, D.R.; Berman, K.F. Brain-derived neurotrophic factor (BDNF) ValMet polymorphism differentially predicts hippocampal function in medication-free patients with schizophrenia. Mol. Psychiatry 2013, 18, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Sham, P.C.; Vallada, H.; Xie, T.; Tang, X.; Murray, R.M.; Liu, X.; Collier, D.A. Preferential transmission of the high activity allele of COMT in schizophrenia. Psychiatr. Genet. 1996, 6, 131–133. [Google Scholar] [CrossRef] [PubMed]
- De Chaldée, M.; Laurent, C.; Thibaut, F.; Martinez, M.; Samolyk, D.; Petit, M.; Campion, D.; Mallet, J. Linkage disequilibrium on the COMT gene in French schizophrenics and controls. Am. J. Med. Genet. 1999, 88, 452–457. [Google Scholar] [CrossRef]
- Kunugi, H.; Vallada, H.P.; Sham, P.C.; Hoda, F.; Arranz, M.J.; Li, T.; Nanko, S.; Murray, R.M.; McGuffin, P.; Owen, M.; et al. Catechol-O-methyltransferase polymorphisms and schizophrenia: A transmission disequilibrium study in multiply affected families. Psychiatr. Genet. 1997, 7, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Doherty, J.L. What can we learn from the high rates of schizophrenia in people with 22q11.2 deletion syndrome? World Psychiatry 2016, 15, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Goldberg, T.E.; Kolachana, B.S.; Callicott, J.H.; Mazzanti, C.M.; Straub, R.E.; Goldman, D.; Weinberger, D.R. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 6917–6922. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Hashimoto, R.; Mori, T.; Nemoto, K.; Moriguchi, Y.; Iida, H.; Noguchi, H.; Nakabayashi, T.; Hori, H.; Ohmori, M. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain 2006, 129, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.C.; Wassink, T.H.; O’Leary, D.S.; Sheffield, V.C.; Andreasen, N.C. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: Working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol. Psychiatry 2005, 10, 287–298. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.M.; Baig, B.J.; Hall, J.; Job, D.; Whalley, H.C.; Lymer, G.K.S.; Moorhead, T.W.J.; Owens, D.G.C.; Miller, P.; Porteous, D. Relationship of catechol-O-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol. Psychiatry 2007, 61, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.D.; Zuchner, S.; Payne, M.E.; Messer, D.F.; Doty, T.J.; MacFall, J.R.; Beyer, J.L.; Krishnana, K.R.R. The COMT Val158Met polymorphism and temporal lobe morphometry in healthy adults. Psychiatry Res. 2007, 155, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.; Liu, J.; Hass, J.; White, T.; Scholz, M.; Roessner, V.; Gollub, R.; Calhoun, V.D.; Ehrlich, S. MB-COMT promoter DNA methylation is associated with working-memory processing in schizophrenia patients and healthy controls. Epigenetics 2014, 9, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Lipsky, R.; Mentschel, C.; Robinson, D.; Gunduz-Bruce, H.; Sevy, S.; Ashtari, M.; Napolitano, B.; Bilder, R.M.; Kane, J.M.; et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol. Psychiatry 2005, 10, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Dutt, A.; McDonald, C.; Dempster, E.; Prata, D.; Shaikh, M.; Williams, I.; Schulze, K.; Marshall, N.; Walshe, M.; Allin, M.; et al. The effect of COMT, BDNF, 5-HTT, NRG1 and DTNBP1 genes on hippocampal and lateral ventricular volume in psychosis. Psychol. Med 2009, 39, 1783–1797. [Google Scholar] [CrossRef] [PubMed]
- Trost, S.; Platz, B.; Usher, J.; Scherk, H.; Wobrock, T.; Ekawardhani, S.; Meyer, J.; Reith, W.; Falkai, P.; Gruber, O. The DTNBP1 (dysbindin-1) gene variant rs2619522 is associated with variation of hippocampal and prefrontal grey matter volumes in humans. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 263, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bis, J.C.; DeCarli, C.; Smith, A.V.; van der Lijn, F.; Crivello, F.; Fornage, M.; Debette, S.; Shulman, J.M.; Schmidt, H.; Srikanth, V. Common variants at 12q14 and 12q24 are associated with hippocampal volume. Nat. Genet. 2012, 44, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, T.; Bousman, C.A.; Liu, C.; Everall, I.P. Schizophrenia genetics in the genome-wide era: A review of Japanese studies. NPJ Schizophr. 2017, 30, 27. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.J. Recent genetic findings in schizophrenia and their therapeutic relevance. J. Psychopharmacol. 2015, 29, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Volk, D.W.; Chitrapu, A.; Edelson, J.R.; Roman, K.M.; Moroco, A.E.; Lewis, D.A. Molecular mechanisms and timing of cortical immune activation in schizophrenia. Am. J. Psychiatry 2015, 172, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Miki, T.; Shoji, H.; Nishi, M.; Takeshima, H.; Miyakawa, T.; Mori, Y. Comprehensive behavioral analysis of voltage-gated calcium channel beta-anchoring and -regulatory protein knockout mice. Front. Behav. Neurosci. 2015, 9, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frodl, T.; Amico, F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Takao, K.; Kobayashi, K.; Hagihara, H.; Ohira, K.; Shoji, H.; Hattori, D.; Koshimizu, H.; Umemori, J.; Toyama, K.; Nakamura, H.K.; et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology 2013, 38, 1409–1425. [Google Scholar] [CrossRef] [PubMed]
- Van Kesteren, C.F.; Gremmels, H.; de Witte, L.D.; Hol, E.M.; Van Gool, A.R.; Falkai, P.G.; Kahn, R.S.; Sommer, I.E. Immune involvement in the pathogenesis of schizophrenia: A meta-analysis on postmortem brain studies. Transl. Psychiatry 2017, 7, e1075. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, M.C.; O’Donovan, M.C.; Craddock, N.; Norton, N.; Williams, H.; Peirce, T.; Moskvina, V.; Nikolov, I.; Hamshere, M.; Carroll, L.; et al. Identification of novel schizophrenia loci by genome-wide association and follow-up. Nat. Genet. 2008, 40, 1053–1055. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, H.; Ophoff, R.A.; Steinberg, S.; Andreassen, O.A.; Cichon, S.; Rujescu, D.; Werge, T.; Pietiläinen, O.P.H.; Mors, O.; Mortensen, P.B.; et al. Common variants conferring risk of schizophrenia. Nature 2009, 460, 744–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, J.; Levinson, D.F.; Duan, J.; Sanders, A.R.; Zheng, Y.; Pe’er, I.; Dudbridge, F.; Holmans, P.A.; Whittemore, A.S.; Mowry, B.J.; et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature 2009, 460, 753–757. [Google Scholar] [CrossRef] [PubMed]
- International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia that overlaps with bipolar disorder. Nature 2009, 460, 748–752. [Google Scholar]
- Ikeda, M.; Aleksic, B.; Kinoshita, Y.; Okochi, T.; Kawashima, K.; Kushima, I.; Ito, Y.; Nakamura, Y.; Kishi, T.; Okumura, T.; et al. Genome-wide association study of schizophrenia in a Japanese population. Biol. Psychiatry 2011, 69, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Psychiatric Genome-Wide Association Study Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011, 43, 969–976. [Google Scholar] [Green Version]
- Rietschel, M.; Mattheisen, M.; Degenhardt, F.; GROUP Investigators; Genetic Risk and Outcome in Psychosis (GROUP Investigators); Mühleisen, T.W.; Kirsch, P.; Esslinger, C.; Herms, S.; Demontis, D.; et al. Association between genetic variation in a region on chromosome 11 and schizophrenia in large samples from Europe. Mol. Psychiatry 2012, 17, 906–917. [Google Scholar] [PubMed]
- Irish Schizophrenia Genomics Consortium; Wellcome Trust Case Control Consortium. Genome-wide association study implicates HLA-C*01:02 as a risk factor at the major histocompatibility complex locus in schizophrenia. Biol. Psychiatry 2017, 72, 620–628. [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet 2013, 381, 1371–1379. [Google Scholar]
- Hamshere, M.L.; Walters, J.T.; Smith, R.; Richards, A.L.; Green, E.; Grozeva, D.; Jones, I.; Forty, L.; Jones, L.; Gordon-Smith, K. Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol. Psychiatry 2013, 18, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, J.H.; Song, G.G. Pathway analysis of a genome-wide association study in schizophrenia. Gene 2013, 525, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lencz, T.; Guha, S.; Liu, C.; Rosenfeld, J.; Mukherjee, S.; DeRosse, P.; John, M.; Cheng, L.; Zhang, C.; Badner, J.A.; et al. Genome-wide association study implicates NDST3 in schizophrenia and bipolar disorder. Nat. Commun. 2013, 4, 2739. [Google Scholar] [CrossRef] [PubMed]
- Ripke, S.; O’Dushlaine, C.; Chambert, K.; Moran, J.L.; Kähler, A.K.; Akterin, S.; Bergen, S.E.; Collins, A.L.; Crowley, J.J.; Fromer, M.; et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 2013, 45, 1150–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [Green Version]
- Kantojärvi, K.; Liuhanen, J.; Saarenpää-Heikkilä, O.; Satomaa, A.L.; Kylliäinen, A.; Pölkki, P.; Jaatela, J.; Toivola, A.; Milani, L.; Himanen, S.L.; et al. Variants in calcium voltage-gated channel subunit Alpha1 C-gene (CACNA1C) are associated with sleep latency in infants. PLoS ONE 2017, 9, e0180652. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Mo, Y.; Sun, X.; Yu, H.; Li, H.; Wu, L.; Li, M. The impact of CACNA1C allelic variation on regional gray matter volume in Chinese population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2016, 171B, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, T.M.; Foley, S.; Tansey, K.E.; Linden, D.E.; Caseras, X. CACNA1C risk variant is associated with increased amygdala volume. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Sumner, J.A.; Sheridan, M.A.; Drury, S.S.; Esteves, K.C.; Walsh, K.; Koenen, K.C.; McLaughlin, K.A. Variation in CACNA1C is Associated with Amygdala Structure and Function in Adolescents. J. Child Adolesc. Psychopharmacol. 2015, 25, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, J.R.; Patel, V.; Jones, T.; Jung, R.; Payaknait, N.; Qualls, C.; Canive, J.M.; Liu, J.; Perrone-Bizzozero, N.I.; Calhoun, V.D.; et al. Risk-Conferring Glutamatergic Genes and Brain Glutamate Plus Glutamine in Schizophrenia. Front. Psychiatry 2017, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Pöhlmann, M.L.; Richter, J.S.; Mehta, D.; Czamara, D.; Metzger, M.W.; Dine, J.; Bedenk, B.T.; Hartmann, J.; Wagner, K.V.; et al. Cross-disorder risk gene CACNA1C differentially modulates susceptibility to psychiatric disorders during development and adulthood. Mol. Psychiatry 2017. [Google Scholar] [CrossRef] [PubMed]
- Cheli, V.T.; Santiago González, D.A.; Namgyal Lama, T.; Spreuer, V.; Handley, V.; Murphy, G.G.; Paez, P.M. Conditional Deletion of the L-Type Calcium Channel Cav1.2 in Oligodendrocyte Progenitor Cells Affects Postnatal Myelination in Mice. J. Neurosci. 2016, 36, 10853–10869. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.T.; Rujescu, D.; Franke, B.; Giegling, I.; Vásquez, A.A.; Hargreaves, A.; Russo, G.; Morris, D.W.; Hoogman, M.; Da Costa, A.; et al. The role of the major histocompatibility complex region in cognition and brain structure: A schizophrenia GWAS follow-up. Am. J. Psychiatry 2013, 170, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Corvin, A.; Morris, D.W. Genome-wide association studies: Findings at the major histocompatibility complex locus in psychosis. Biol. Psychiatry 2014, 75, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Isobe, M.; Tanigaki, K.; Muraki, K.; Miyata, J.; Takemura, A.; Sugihara, G.; Takahashi, H.; Aso, T.; Fukuyama, H.; Hazama, M.; et al. Polymorphism within a Neuronal Activity-Dependent Enhancer of NgR1 Is Associated with Corpus Callosum Morphology in Humans. Mol. Neuropsychiatry 2015, 1, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Bomba, L.; Walter, K.; Soranzo, N. The impact of rare and low-frequency genetic variants in common disease. Genome Biol. Apr. 2017, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-F.; Goldstein, D.B.; Angrist, M.; Cavalleri, G. Personalized Medicine and Human Genetic Diversity. Cold Spring Harbor Perspect. Med. 2014, 4, a008581. [Google Scholar] [CrossRef] [PubMed]
- Bassett, A.S.; Lowther, C.; Merico, D.; Costain, G.; Chow, E.W.C.; van Amelsvoort, T.; McDonald-McGinn, D.; Gur, R.E.; Swillen, A.; Van den Bree, M.; et al. Genome-Wide Copy Number Variation and Expression of Schizophrenia in 22q11.2 Deletion Syndrome. Am. J. Psychiatry 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.R.; Howrigan, D.P.; Merico, D.; Thiruvahindrapuram, B.; Wu, W.; Greer, D.S.; Antaki, D.; Shetty, A.; Holmans, P.A.; Pinto, D.; et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat. Genet. 2017, 49, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Terwisscha van Scheltinga, A.; Bakker, S.; van Haren, N.; Buizer-Voskamp, J.; Boos, H.; Vorstman, J.; Cahn, W.; Hulshoff Pol, H.; Ophoff, R.; Kahn, R. Association study of copy number variants with brain volume in schizophrenia patients and healthy controls. Psychiatry Res. 2012, 200, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.M.; Stein, J.L.; Medland, S.E.; Hibar, D.P.; Vasquez, A.A.; Renteria, M.E.; Toro, R.; Jahanshad, N.; Schumann, G.; Franke, B.; et al. The ENIGMA Consortium: Large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014, 8, 153–182. [Google Scholar] [PubMed]
- Wang, L.; Alpert, K.I.; Calhoun, V.D.; Cobia, D.J.; Keator, D.B.; King, M.D.; Kogana, A.; Landis, D.; Tallis, M.; Turner, M.D.; et al. SchizConnect: Mediating Neuroimaging Databases on Schizophrenia and Related Disorders for Large-Scale Integration. NeuroImage 2016, 124, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Hibar, D.P.; Adams, H.H.H.; Jahanshad, N.; Chauhan, G.; Stein, J.L.; Hofer, E.; Renteria, M.E.; Bis, J.C.; Arias-Vasquez, A.; Ikram, M.K.; et al. Novel genetic loci associated with hippocampal volume. Nat. Commun. 2017, 8, 13624. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Zhang, J.; Ahn, M.; Jha, S.; Crowley, J.J.; Szatkiewicz, J.; Li, T.; Zou, F.; Zhu, H.; Hibar, D.; et al. Genome-wide association analysis identifies common variants influencing infant brain volumes. Transl. Psychiatry 2017, 7, e1188. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Martins-de-Souza, D.; Akbarian, S.; Cassoli, J.S.; Ehrenreich, H.; Fischer, A.; Fonteh, A.; Gattaz, W.F.; Gawlik, M.; Gerlach, M.; et al. Consensus paper of the WFSBP Task Force on Biological Markers: Criteria for biomarkers and endophenotypes of schizophrenia part II: Cognition, neuroimaging and genetics. World J. Biol. Psychiatry 2016, 17, 406–428. [Google Scholar] [CrossRef] [PubMed]
- Kambeitz, J.; Kambeitz-Ilankovic, L.; Leucht, S.; Wood, S.; Davatzikos, C.; Malchow, B.; Falkai, P.; Koutsouleris, N. Detecting neuroimaging biomarkers for schizophrenia: A meta-analysis of multivariate pattern recognition studies. Neuropsychopharmacology 2015, 40, 1742–1751. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Duan, J.; Lin, D.; Shugart, Y.Y.; Calhoun, V.; Wang, Y.P. Sparse representation based biomarker selection for schizophrenia with integrated analysis of fMRI and SNPs. Neuroimage 2014, 102, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lin, D.; Calhoun, V.D.; Wang, Y.P. Integration of SNPs-FMRI-methylation data with sparse multi-CCA for schizophrenia study. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 2016, 3310–3313. [Google Scholar]
- Doan, N.T.; Kaufmann, T.; Bettella, F.; Jørgensen, K.N.; Brandt, C.L.; Moberget, T.; Alnæs, D.; Douaud, G.; Duff, E.; Djurovic, S.; et al. Distinct multivariate brain morphological patterns and their added predictivevalue with cognitive and polygenic risk scores in mental disorders. Neuroimage Clin. 2017, 15, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, K.S.; Arakelian, C.R.; van Erp, T.G.M. Heritability of Hippocampal Formation Sub-region Volumes. J. Neurol. Neurosci. 2016, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Alural, B.; Genc, S.; Haggarty, S.J. Diagnostic and therapeutic potential of microRNAs in neuropsychiatric disorders: Past, present, and future. Prog. Neuro-Psychopharmacol. Boil. Psychiatry 2017, 73, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Carvalho, M.; Wu, W.C.; Cummings, K.A.; Clem, R.L. Optogenetic Examination of Prefrontal-Amygdala Synaptic Development. J. Neurosci. 2017, 37, 2976–2985. [Google Scholar] [CrossRef] [PubMed]
- Riga, D.; Matos, M.R.; Glas, A.; Smit, A.B.; Spijker, S.; Van den Oever, M.C. Optogenetic dissection of medial prefrontal cortex circuitry. Front. Syst. Neurosci. 2014, 8, 230. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Gupta, C.N.; Chen, J.; Patel, V.; Calhoun, V.D.; Ehrlich, S.; Wang, L.; Bustillo, J.R.; Perrone-Bizzozero, N.I.; Turner, J.A. Polymorphisms in MIR137HG and microRNA-137-regulated genes influence gray matter structure in schizophrenia. Transl. Psychiatry 2016, 6, e724. [Google Scholar] [CrossRef] [PubMed]
- Erk, S.; Mohnke, S.; Ripke, S.; Lett, T.A.; Veer, I.M.; Wackerhagen, C.; Grimm, O.; Romanczuk-Seiferth, N.; Degenhardt, F.; Tost, H.; et al. Functional neuroimaging effects of recently discovered genetic risk loci for schizophrenia and polygenic risk profile in five RDoC subdomains. Transl. Psychiatry 2017, 7, e997. [Google Scholar] [CrossRef] [PubMed]
- Reus, L.M.; Shen, X.; Gibson, J.; Wigmore, E.; Ligthart, L.; Adams, M.J.; Davies, G.; Cox, S.R.; Hagenaars, S.P.; Bastin, M.E.; et al. Association of polygenic risk for major psychiatric illness with subcortical volumes and white matter integrity in UK Biobank. Sci. Rep. 2017, 7, 42140. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.H.; Hibar, D.P.; Chouraki, V.; Stein, J.L.; Nyquist, P.A.; Rentería, M.E.; Trompet, S.; Arias-Vasquez, A.; Seshadri, S.; Desrivières, S.; et al. Novel genetic loci underlying human intracranial volume identified through genome-wide association. Nat. Neurosci. 2016, 19, 1569–1582. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arslan, A. Mapping the Schizophrenia Genes by Neuroimaging: The Opportunities and the Challenges. Int. J. Mol. Sci. 2018, 19, 219. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010219
Arslan A. Mapping the Schizophrenia Genes by Neuroimaging: The Opportunities and the Challenges. International Journal of Molecular Sciences. 2018; 19(1):219. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010219
Chicago/Turabian StyleArslan, Ayla. 2018. "Mapping the Schizophrenia Genes by Neuroimaging: The Opportunities and the Challenges" International Journal of Molecular Sciences 19, no. 1: 219. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010219




