Mechanical Activation of Adipose Tissue and Derived Mesenchymal Stem Cells: Novel Anti-Inflammatory Properties
Abstract
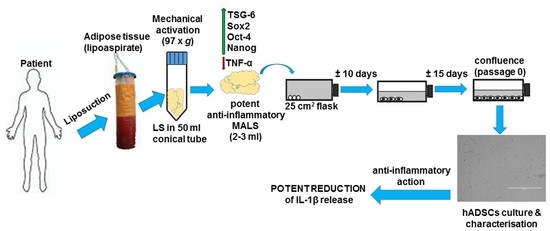
:1. Introduction
2. Results
2.1. Tissue Mechanical Activation
2.2. Preparation of Mesenchymal Stem Cells Cultures
2.3. Adipogenic and Osteogenic Differentiation
2.4. hADSCs Isolated from Mechanically Activated Adipose Tissue Show Strong Anti-Inflammatory Features
3. Discussion
4. Materials and Methods
4.1. Tissue Mechanical Activation and Informed Consent
4.2. Cell Cultures
4.3. Cell Growth Analysis
4.4. G-Banding Karyotype Analyses
4.5. Flow Cytofluorimetry: Immunophenotipic Characterization
4.6. In Vitro Differentiation
4.7. THP-1 Cells Culture and Activation with Lipopolysaccharides
4.8. Immunocytochemistry
4.9. RNA Extraction and qRT-PCR Analyses
4.9.1. Gene Expression in Adipose Tissues
4.9.2. Gene Expression Profile in Cells
- 18S F: AGTACGCACGGGCCGGTACAGTGAACTGCG;
- 18S R: CGGGTTGGTTTTGATCTGATAAATGCACGC;
- GAPDH F: CTTTTGCGTCGCCAG;
- GAPDH R: TTGATGGCAACAATATCCAC;
- IL1β F: ACAGATGAAGTGCTCCTTCCA;
- IL1β R: GTCGGAGATTCGTAGCTGGAT;
- TNF-α F: CACTGAAAGCATGATCCGGGACGTGGAGCT;
- TNF-α R: TCTTCCCTCTGGGGGCCGATCACTCCAAAG;
- TSG-6 F: CATCTTAATTTACTTATTTCTCTTGCTATG;
- TSG-6 R: TCTGGCTGCCTCTAGCTGCTTGTAAGTTGC;
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Kales, A.; Tyson, K.; Chrousos, G.P. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: Role of sleep disturbance and obesity. J. Clin. Endocrinol. Metab. 1997, 82, 1313–1316. [Google Scholar] [CrossRef] [PubMed]
- Perreault, M.; Marette, A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat. Med. 2001, 7, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Yamamoto, K.; Pandey, M.; Loskutoff, D.J. Elevated expression of transforming growth factor-β in adipose tissue from obese mice. Mol. Med. 1997, 3, 37–48. [Google Scholar] [PubMed]
- Visser, M.; Bouter, L.M.; McQuillan, G.M.; Wener, M.H.; Harris, T.B. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999, 282, 2131–2135. [Google Scholar] [CrossRef] [PubMed]
- Sartipy, P.; Loskutoff, D.J. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc. Natl. Acad. Sci. USA 2003, 100, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Yamamoto, K.; Loskutoff, D.J. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-α and lipopolysaccharide. J. Clin. Investig. 1996, 97, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Pandey, M.; Loskutoff, D.J. Tissue factor gene expression in the adipose tissues of obese mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7591–7596. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Pannacciulli, N. Coagulation and fibrinolysis abnormalities in obesity. J. Endocrinol. Investig. 2002, 25, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Bunkin, D.A.; Greenberg, A.S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6, depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998, 83, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.P.; Salanga, C.L.; Johns, S.C.; Valdambrini, E.; Fuster, M.M.; Milner, C.M.; Day, A.J.; Handel, T.M. The anti-inflammatory protein TSG-6 regulates chemokine function by inhibiting chemokine/glycosaminoglycan interactions. J. Biol. Chem. 2016, 29, 12627–12640. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Mark, A.; Moorman, D.W.; Simonetti, S.C.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Bunnell, B.A.; Chiu, E.S.; Guilak, F. Taking stem cells beyond discovery: A milestone in the reporting of regulatory requirements for cell therapy. Stem Cells Dev. 2011, 20, 1295–1296. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Kern, S.; Klüter, H.; Eichler, H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 2004, 22, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–229. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Silvestre, J.S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of human adipose lineage cells towards endothelial cells: Physiological and therapeutic perspectives. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Naderi, N.; Wilde, C.; Haque, T.; Francis, W.; Seifalian, A.M.; Thornton, C.A.; Xia, Z.; Whitaker, I.S. Adipogenic differentiation of adiposederived stem cells in a 3-dimensional spheroid culture (microtissue): Implications for the reconstructive surgeon. J. Plast. Reconstr. Aesthet. Surg. 2014, 67, 1726–1734. [Google Scholar] [CrossRef] [PubMed]
- Ude, C.C.; Sulaiman, S.B.; Min-Hwei, N.; Hui-Cheng, C.; Ahmad, J.; Yahaya, N.M.; Saim, A.B.; Idrus, R.B. Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS ONE 2004, 9, e98770. [Google Scholar] [CrossRef] [PubMed]
- Rehmam, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.K.; Katz, A.J. Review of the adipose derived stem cell secretome. Biochimie 2013, 95, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Eto, H.; Aoi, N.; Kato, H.; Araki, J.M.; Doi, K.; Higashino, T.; Yoshimura, K. Adipose tissue remodeling under ischemia: Death of adipocytes and activation of stem/progenitor cells. Plast. Reconstr. Surg. 2010, 126, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Kato, H.; Suga, H.; Araki, J.M.; Doi, K.; Higashino, T.; Yoshimura, K. The fate of adipocytes after nonvascularized fat grafting: Evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Mineda, K.; Eto, H.; Doi, K.; Kuno, S.; Kinoshita, K.; Kanayama, K.; Yoshimura, K. Degeneration, regeneration and cicatrization after fat grafting: Dynamic total tissue remodeling during the first 3 months. Plast. Reconstr. Surg. 2014, 133, 303e–313e. [Google Scholar] [PubMed]
- Vogel, V.; Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006, 7, 265–275. [Google Scholar] [CrossRef] [PubMed]
- El Haj, A.J.; Walker, L.M.; Preston, M.R.; Publicover, S.J. Mechanotransduction pathways in bone: Calcium fluxes and the role of voltage-operated calcium channels. Med. Biol. Eng. Comput. 1999, 37, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Collin, O.; Chowdhury, F.; Tay, B.; Ouyang, M.; Wang, Y.; Wang, N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc. Natl. Acad. Sci. USA 2008, 105, 6626–6631. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.M.; Publicover, S.J.; Preston, M.R.; Said Ahmed, M.A.; El Haj, A.J. Calcium-channel activation and matrix protein upregulation in bone cells in response to mechanical strain. J. Cell. Biochem. 2000, 79, 648–661. [Google Scholar] [CrossRef]
- Venkatesan, I.K.; Pulford, S.; Mogilner, A.; Shivashankar, G.V. Mechanical activation of cells induces chromatin remodeling preceding MKL nuclear transport. Biophys. J. 2012, 103, 1416–1428. [Google Scholar]
- Chen, L.J.; Wei, S.Y.; Chiu, J.J. Mechanical regulation of epigenetics in vascular biology and pathology. J. Cell. Mol. Med. 2013, 17, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tytell, J.D.; Ingber, D.E. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 2009, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.J.; Metzger, P.; Trengove, N.; Lai, C.T.; Filgueira, L.; Blancafort, P.; et al. Breast milk is a novel source of stem cells with multilineage differentiation potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Karaöz, E.; Okçu, A.; Gacar, G.; Saglam, O.; Yürüker, S.; Kenar, H.A. Comprehensive characterization study of human bone marrow MSCs with an emphasis on molecular and ultrastructural properties. J. Cell. Physiol. 2011, 226, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Lehmann, T.; Cai, H.; Stolz, D.B.; Strom, S.C. Stem cell characteristics of amniotic epithelial cells. Stem Cells 2005, 23, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Carelli, S.; Messaggio, F.; Canazza, A.; Hebda, D.M.; Caremoli, F.; Latorre, E.; Grimoldi, M.G.; Colli, M.; Bulfamante, G.; Tremolada, C. Characteristics and properties of mesenchymal stem cells derived from microfragmented adipose tissue. Cell Transplant. 2015, 24, 1233–1252. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Benhaim, P.; Hedrick, M.H.; Fraser, J.K. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, F.P.; Corrado, A.; Grano, M.; Quarta, L.; Colucci, S.; Melillo, N. Osteocalcin synthesis by human osteoblasts from normal and osteoarthritic bone after vitamin D3 stimulation. Clin. Rheumatol. 2004, 23, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Concise review: Two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem Cells 2013, 31, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Interleukin-1β, Interleukin-18, and the Interleukin-1β Converting Enzyme. Ann. N. Y. Acad. Sci. 1998, 856, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kostura, M.J.; Tocci, M.J.; Limjuco, G.; Chin, J.; Cameron, P.; Hillman, A.G.; Chartrain, N.A.; Schmidt, J.A. Identification of a monocyte specific pre-interleukin 1β convertase activity. Proc. Natl. Acad. Sci. USA 1989, 86, 5227–5231. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Al Shahrani, M.; Al-Habib, M.; Tanaka, T.; Huang, G.T.J. Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J. Endod. 2011, 37, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef]
- Avilion, A.A.; Nicolis, S.K.; Pevny, L.H.; Perez, L.; Vivian, N.; Lovell-Badge, R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003, 17, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Go, M.J.; Takenaka, C.; Ohgushi, H. Forced expression of Sox2 or Nanog in human bone marrow derived mesenchymal stem cells maintains their expansion and differentiation capabilities. Exp. Cell Res. 2008, 314, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yoon, J.G.; Li, L.; Yu, W.; Shao, J.; Hua, D.; Zheng, S.; Hood, L.; Goodlett, D.R.; Foltz, G.; et al. The SOX2 response program in glioblastoma multiforme: An integrated ChIP-seq, expression microarray, and microRNA analysis. BMC Genom. 2011, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Wisnieswki, H.G.; Maier, L.; Lotz, M.; Lee, S.; Klampfer, L.; Lee, T.H.; Vilcek, J. TSG6: A TNF-, IL-1-, LPS-inducible secreted glycoprotein associated with arthritis. J. Immunol. 1993, 151, 6593–6601. [Google Scholar]
- Bardos, T.; Kamath, R.V.; Mikecz, K.; Glant, T.T. Anti-inflammatory and condroprotective effect of TSG6 in murine models of experimental arthritis. Am. J. Pathol. 2001, 159, 1711–1721. [Google Scholar] [CrossRef]
- Kato, T.; Okumi, M.; Tanemura, M.; Yazawa, K.; Kakuta, Y.; Yamanaka, K.; Tsutahara, K.; Doki, Y.; Mori, M.; Takahara, S.; Nonomura, N. Adipose tissue-derived stem cells suppress acute cellular rejection by TSG6 and CD44 interaction in rat kidney transplantation. Transplantation 2014, 98, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegeman, A.; Schat, Z.S.; Treiber, N.; Rojewski, M.; Seitz, A.; Scolz, N.; Drecelen, L.; et al. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis. J. Investig. Dermatol. 2014, 134, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Chen, C.S. Cell adhesion and mechanical stimulation in the regulation of mesenchymal stem cell differentiation. J. Cell. Mol. Med. 2013, 17, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Uzer, G.; Fuchs, R.K.; Rubin, J.; Thompson, W.R. Plasma and Nuclear Membranes Convey Mechanical Information to Regulate Mesenchymal Stem Cell Lineage. Stem Cells 2016, 34, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, I.; Nuckolls, G.H.; Takahashi, K.; Tanaka, O.; Semba, I.; Dashner, R.; Shum, L.; Slavkin, H.C. Compressive force promotes sox9, type II collagen and aggrecan and inhibits IL-1β expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J. Cell Sci. 1998, 111, 2067–2076. [Google Scholar] [PubMed]
- Huang, C.Y.; Hargar, K.L.; Frost, L.E.; Sun, Y.; Cheung, H.S. Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 2004, 22, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.N.; Yoon, H.H.; Seo, Y.K.; Park, J.K. Effect of mechanical stimulation on the differentiation of cord stem cells. Connect. Tissue Res. 2012, 53, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Hyun, I.; Lindvall, O.; Ahrlund-Richter, L.; Cattaneo, E.; Cavazzana-Calvo, M.; Cossu, G.; De Luca, M.; Fox, I.J.; Gerstle, C.; Goldstein, R.A.; et al. New ISSCR guidelines underscore major principles for responsible translational stem cell research. Cell Stem Cell 2008, 3, 607–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Xiong, W.; Chen, Y.; Ma, Q.; Ma, J.; Ge, Y.; Han, D. Evaluation on the phagocytosis of apoptotic spermatogenic cells by Sertoli cells in vitro through detecting lipid droplet formation by Oil Red O staining. Reproduction 2006, 132, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Meyers, V.E.; Zayzafoon, M.; Douglas, J.T.; McDonald, J.M. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. J. Bone Miner. Res. 2005, 1858–1866. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carelli, S.; Colli, M.; Vinci, V.; Caviggioli, F.; Klinger, M.; Gorio, A. Mechanical Activation of Adipose Tissue and Derived Mesenchymal Stem Cells: Novel Anti-Inflammatory Properties. Int. J. Mol. Sci. 2018, 19, 267. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010267
Carelli S, Colli M, Vinci V, Caviggioli F, Klinger M, Gorio A. Mechanical Activation of Adipose Tissue and Derived Mesenchymal Stem Cells: Novel Anti-Inflammatory Properties. International Journal of Molecular Sciences. 2018; 19(1):267. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010267
Chicago/Turabian StyleCarelli, Stephana, Mattia Colli, Valeriano Vinci, Fabio Caviggioli, Marco Klinger, and Alfredo Gorio. 2018. "Mechanical Activation of Adipose Tissue and Derived Mesenchymal Stem Cells: Novel Anti-Inflammatory Properties" International Journal of Molecular Sciences 19, no. 1: 267. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010267