Synergistic Effects of Copper Sites on Apparent Stability of Multicopper Oxidase, Fet3p
Abstract
:1. Introduction
2. Results
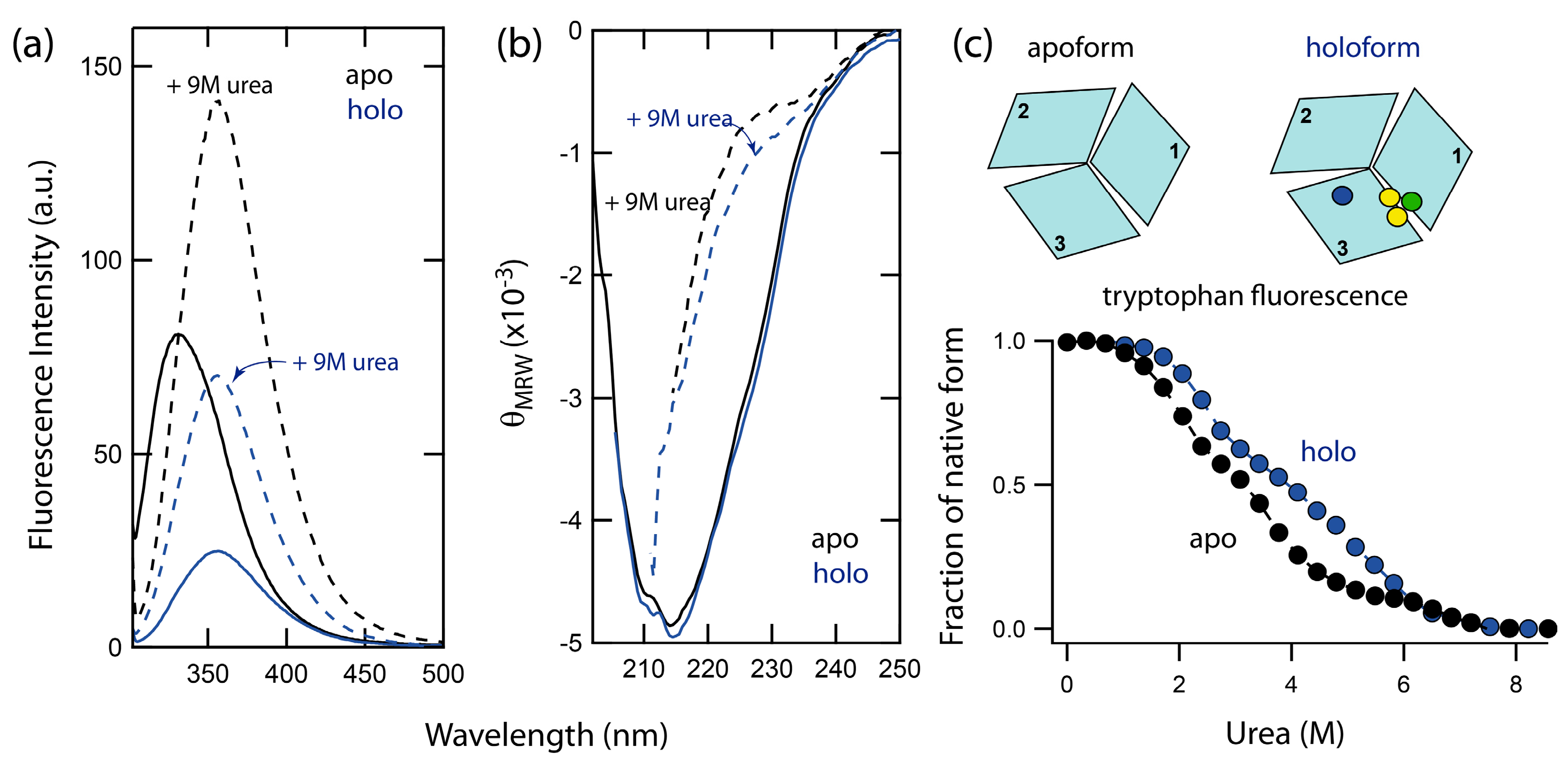
2.1. Spectral Properties of Folded and Urea-Denatured Wild-Type Fet3p
2.2. Unfolding Reactions of Apo- and Holo-Forms of Fet3p
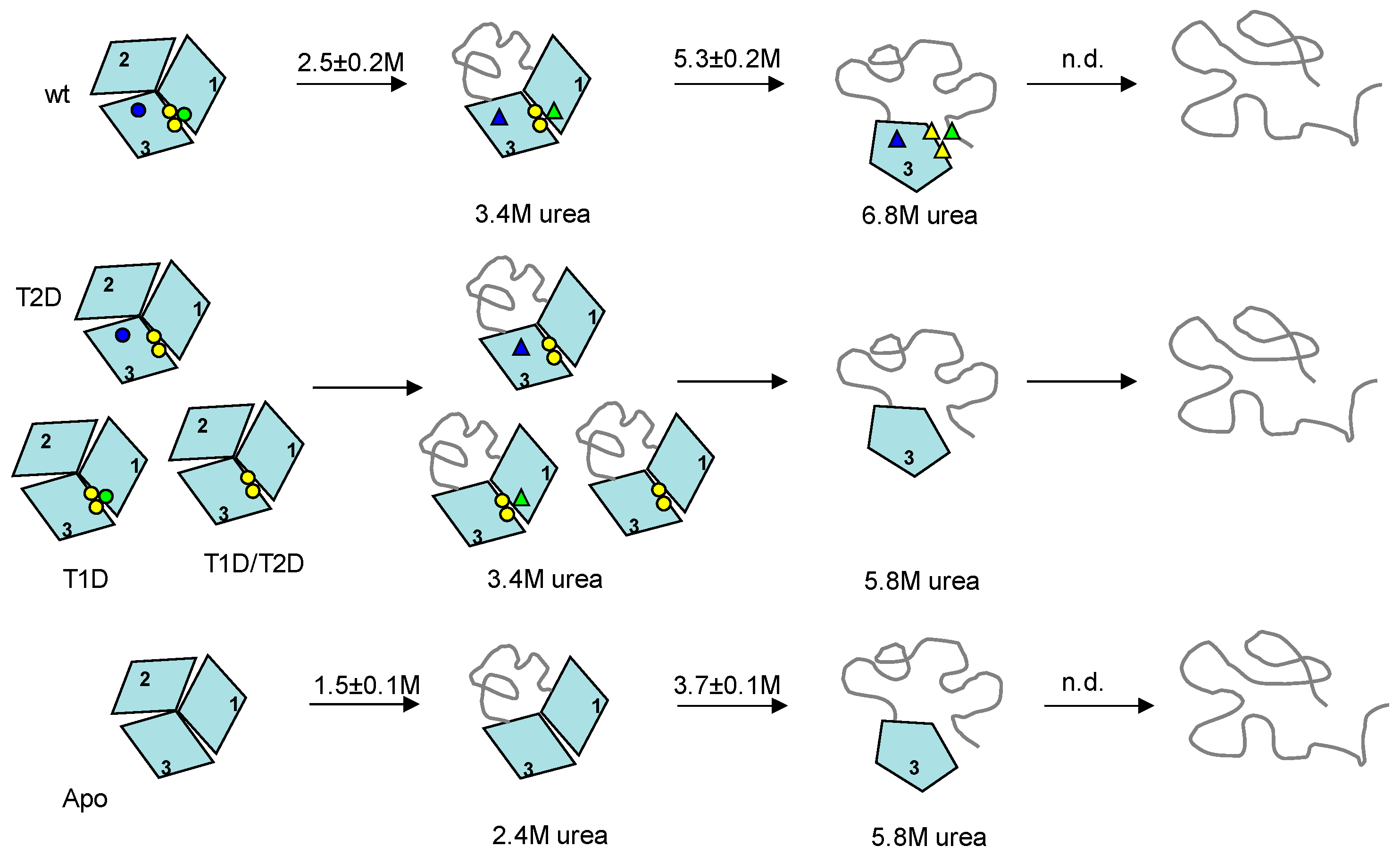
2.3. Probing Partially-Metallated Fet3p Variants
2.3.1. Holo-Form of Wild-Type Fet3p
2.3.2. T2D Variant of Fet3p
2.3.3. T1D Variant of Fet3p
2.3.4. T1D/T2D Variant of Fet3p
2.3.5. Apo-Form of Wild-Type Fet3p
2.3.6. Phase Diagram Analysis of Fet3p Variant Data
3. Discussion
4. Materials and Methods
4.1. Protein Preparation
4.2. Isothermal Unfolding Experiments
4.3. Spectroscopy
4.4. Activity Assay
4.5. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taylor, A.B.; Stoj, C.S.; Ziegler, L.; Kosman, D.J.; Hart, P.J. The copper-iron connection in biology: Structure of the metallo-oxidase Fet3p. Proc. Natl. Acad. Sci. USA 2005, 102, 15459–15464. [Google Scholar] [CrossRef] [PubMed]
- Kosman, D.J. Fet3p, ceruloplasmin, and the role of copper in iron metabolism. Adv. Protein Chem. 2002, 60, 221–269. [Google Scholar] [PubMed]
- Sakurai, T.; Kataoka, K. Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase. Chem. Rev. 2007, 7, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Wittung-Stafshede, P. Role of cofactors in folding of the blue-copper protein azurin. Inorg. Chem. 2004, 43, 7926–7933. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Apiyo, D.; Wittung-Stafshede, P. Role of cofactors in metalloprotein folding. Q. Rev. Biophys. 2004, 37, 285–314. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Sedlak, E.; Wittung-Stafshede, P. Role of copper in folding and stability of cupredoxin-like copper-carrier protein CopC. Arch. Biochem. Biophys. 2007, 467, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; D’Alessio, S.; Giartosio, A.; Morpurgo, L.; Avigliano, L. The role of copper in the stability of ascorbate oxidase towards denaturing agents. Eur. J. Biochem. 1990, 190, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Agostinelli, E.; Cervoni, L.; Giartosio, A.; Morpurgo, L. Stability of Japanese-lacquer-tree (Rhus vernicifera) laccase to thermal and chemical denaturation: Comparison with ascorbate oxidase. Biochem. J. 1995, 306 Pt 3, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Augustine, A.J.; Kragh, M.E.; Sarangi, R.; Fujii, S.; Liboiron, B.D.; Stoj, C.S.; Kosman, D.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Spectroscopic studies of perturbed T1 Cu sites in the multicopper oxidases Saccharomyces cerevisiae Fet3p and Rhus vernicifera laccase: Allosteric coupling between the T1 and trinuclear Cu sites. Biochemistry 2008, 47, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, E.; Wittung-Stafshede, P. Discrete roles of copper ions in chemical unfolding of human ceruloplasmin. Biochemistry 2007, 46, 9638–9644. [Google Scholar] [CrossRef] [PubMed]
- Žoldák, G.; Jancura, D.; Sedlák, E. The fluorescence intensities ratio is not a reliable parameter for evaluation of protein unfolding transitions. Protein Sci. 2017, 26, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Burstein, E.A.; Vedenkina, N.S.; Ivkova, M.N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem. Photobiol. 1973, 18, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Stryer, L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J. Mol. Biol. 1965, 13, 482–495. [Google Scholar] [CrossRef]
- Bushmarina, N.A.; Kuznetsova, I.M.; Biktashev, A.G.; Turoverov, K.K.; Uversky, V.N. Partially folded conformations in the folding pathway of bovine carbonic anhydrase II: A fluorescence spectroscopic analysis. Chembiochem 2001, 2, 813–821. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Turoverov, K.K.; Uversky, V.N. Use of the phase diagram method to analyze the protein unfolding-refolding reactions: Fishing out the “invisible” intermediates. J. Proteome Res. 2004, 3, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, E.; Ziegler, L.; Kosman, D.J.; Wittung-Stafshede, P. In vitro unfolding of yeast multicopper oxidase Fet3p variants reveals unique role of each metal site. Proc. Natl. Acad. Sci. USA 2008, 105, 19258–19263. [Google Scholar] [CrossRef] [PubMed]
- Bello, M.; Valderrama, B.; Serrano-Posada, H.; Rudiño-Piñera, E. Molecular dynamics of a thermostable multicopper oxidase from Thermus thermophilus HB27: Structural differences between the apo and holo forms. PLoS ONE 2012, 7, e40700. [Google Scholar] [CrossRef] [PubMed]
- Roulling, F.; Godin, A.; Cipolla, A.; Collins, T.; Miyazaki, K.; Feller, G. Activity-stability relationships revisited in blue oxidases catalyzing electron transfer at extreme temperatures. Extremophiles 2016, 20, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, N.J.; Ralle, M.; Hassett, R.; Kosman, D.J. Spectroscopic analysis of the trinuclear cluster in the Fet3 protein from yeast, a multinuclear copper oxidase. Biochemistry 2000, 39, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Hassett, R.F.; Yuan, D.S.; Kosman, D.J. Spectral and kinetic properties of the Fet3 protein from Saccharomyces cerevisiae, a multinuclear copper ferroxidase enzyme. J. Biol. Chem. 1998, 273, 23274–23282. [Google Scholar] [CrossRef] [PubMed]
- Felsenfeld, G. The determination of cuprous ion in copper proteins. Arch. Biochem. Biophys. 1960, 87, 247–251. [Google Scholar] [CrossRef]
- Schosinsky, K.H.; Lehmann, H.P.; Beeler, M.F. Measurement of ceruloplasmin from its oxidase activity in serum by use of o-dianisidine dihydrochloride. Clin. Chem. 1974, 20, 1556–1563. [Google Scholar] [PubMed]
- Santoro, M.M.; Bolen, D.W. A test of the linear extrapolation of unfolding free energy changes over an extended denaturant concentration range. Biochemistry 1992, 31, 4901–4907. [Google Scholar] [CrossRef] [PubMed]
- Sancho, J. The stability of 2-state, 3-state and more-state proteins from simple spectroscopic techniques... plus the structure of the equilibrium intermediates at the same time. Arch. Biochem. Biophys. 2013, 531, 4–13. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlák, E.; Žoldák, G.; Wittung-Stafshede, P. Synergistic Effects of Copper Sites on Apparent Stability of Multicopper Oxidase, Fet3p. Int. J. Mol. Sci. 2018, 19, 269. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010269
Sedlák E, Žoldák G, Wittung-Stafshede P. Synergistic Effects of Copper Sites on Apparent Stability of Multicopper Oxidase, Fet3p. International Journal of Molecular Sciences. 2018; 19(1):269. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010269
Chicago/Turabian StyleSedlák, Erik, Gabriel Žoldák, and Pernilla Wittung-Stafshede. 2018. "Synergistic Effects of Copper Sites on Apparent Stability of Multicopper Oxidase, Fet3p" International Journal of Molecular Sciences 19, no. 1: 269. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010269




