Tissue Engineering to Improve Immature Testicular Tissue and Cell Transplantation Outcomes: One Step Closer to Fertility Restoration for Prepubertal Boys Exposed to Gonadotoxic Treatments
Abstract
:1. Introduction
2. Results and Discussion
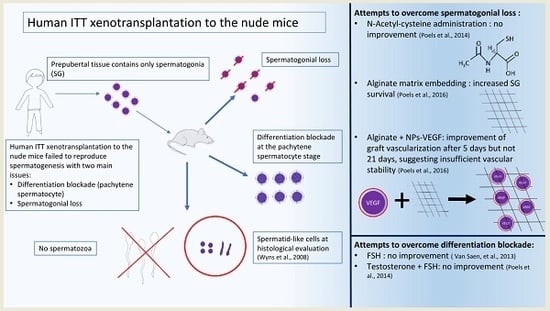
2.1. Lessons Learned from Transplantation of Immature Testicular Tissue Fragments
2.1.1. Transplantation Technique
2.1.2. Transplantation Site
2.1.3. Maturational Stage of Donor Tissue
2.1.4. The Host Environment
2.2. Acting on the Endocrine Environment
2.3. Reducing Ischemia Due to the Avascular Transplantation Procedure
2.4. Using Protective Molecules to Reduce Ischemic Injury
2.5. Challenges to Achieve a Successful Transplantation of Human Immature Testicular Tissue
2.6. Lessons Learned from Transplantation of Spermatogonial Stem Cells (SSCs)
2.7. Challenges to Achieve a Successful Transplantation of Human Immature SSCs
2.8. Lessons Learned from Reports on Cellular and Tissue Encapsulation and Perspectives Using Scaffolds
2.8.1. Cells or Tissues Encapsulation
2.8.2. Use of Scaffolds
2.9. Bioactive Molecules Supplementation Using Nanoparticles
2.10. Tissue-Engineering Applications for Testicular Tissue Transplantation
2.11. Future Directions for Fertility Restoration in Boys Using Transplantation of Prepubertal Cells or Tissues
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SSC | Spermatogonial stem cells |
| ITT | Immature testicular tissue |
| SG | Spermatogonia |
| ST | Seminiferous tubules |
| F/T | Frozen/Thawed |
| FSH | Follicle stimulating Hormone |
| LH | Luteinizing Hormone |
| HcG | Human chorionic Gonadotropin |
| VEGF | Vascular Endothelial Growth Factor |
| PDGF | Platelet Derived Growth Factor |
| FGF | Fibroblast Growth Factor |
| MACS | Magnetic Activated Cell Sorting |
| FACS | Fluorescence Activated Cell Sorting |
References
- Gatta, G.; Zigon, G.; Capocaccia, R.; Coebergh, J.W.; Desandes, E.; Kaatsch, P.; Pastore, G.; Peris-Bonet, R.; Stiller, C.A.; Group, E.W. Survival of european children and young adults with cancer diagnosed 1995–2002. Eur. J. Cancer 2009, 45, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Jahnukainen, K.; Mitchell, R.T.; Stukenborg, J.B. Testicular function and fertility preservation after treatment for haematological cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Curaba, M.; Vanabelle, B.; Van Langendonckt, A.; Donnez, J. Options for fertility preservation in prepubertal boys. Hum. Reprod. Update 2010, 16, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Lukusa, A.K.; Vermylen, C.; Vanabelle, B.; Curaba, M.; Brichard, B.; Chantrain, C.; Dupont, S.; Ferrant, A.; Wyns, C. Bone marrow transplantation or hydroxyurea for sickle cell anemia: Long-term effects on semen variables and hormone profiles. Pediatr. Hematol. Oncol. 2009, 26, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American society of clinical oncology recommendations on fertility preservation in cancer patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Schover, L.R.; Rybicki, L.A.; Martin, B.A.; Bringelsen, K.A. Having children after cancer. A pilot survey of survivors’ attitudes and experiences. Cancer 1999, 86, 697–709. [Google Scholar] [CrossRef]
- Wyns, C.; Collienne, C.; Shenfield, F.; Robert, A.; Laurent, P.; Roegiers, L.; Brichard, B. Fertility preservation in the male pediatric population: Factors influencing the decision of parents and children. Hum. Reprod. 2015, 30, 2022–2030. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, J.P.; Li, Y.; Carlson, C.A.; Gracia, C.R.; Hobbie, W.L.; Miller, V.A.; Mulhall, J.; Shnorhavorian, M.; Brinster, R.L.; Kolon, T.F. Testicular tissue cryopreservation in prepubertal male children: An analysis of parental decision-making. Pediatr. Blood Cancer 2014, 61, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, H.; Repping, S.; van der Veen, F. Parental desire and acceptability of spermatogonial stem cell cryopreservation in boys with cancer. Hum. Reprod. 2007, 22, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Clermont, Y. Kinetics of spermatogenesis in mammals. Arch. Anat. Microsc. Morphol. Exp. 1967, 56, 7–60. [Google Scholar] [PubMed]
- Keros, V.; Hultenby, K.; Borgstrom, B.; Fridstrom, M.; Jahnukainen, K.; Hovatta, O. Methods of cryopreservation of testicular tissue with viable spermatogonia in pre-pubertal boys undergoing gonadotoxic cancer treatment. Hum. Reprod. 2007, 22, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Curaba, M.; Martinez-Madrid, B.; Van Langendonckt, A.; Francois-Xavier, W.; Donnez, J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum. Reprod. 2007, 22, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Curaba, M.; Petit, S.; Vanabelle, B.; Laurent, P.; Wese, J.F.; Donnez, J. Management of fertility preservation in prepubertal patients: 5 years’ experience at the catholic university of louvain. Hum. Reprod. 2011, 26, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Picton, H.M.; Wyns, C.; Anderson, R.A.; Goossens, E.; Jahnukainen, K.; Kliesch, S.; Mitchell, R.T.; Pennings, G.; Rives, N.; Tournaye, H.; et al. A european perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum. Reprod. 2015, 30, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Ohmura, M.; Ohbo, K. The niche for spermatogonial stem cells in the mammalian testis. Int. J. Hematol. 2005, 82, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Van Langendonckt, A.; Wese, F.X.; Donnez, J.; Curaba, M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum. Reprod. 2008, 23, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Hargreaves, H.K.; Chan, W.C.; Brynes, R.K.; Alvarado, C.; Woodard, J.; Ragab, A.H. Sequential testicular biopsies in childhood acute lymphocytic leukemia. Cancer 1986, 57, 1038–1041. [Google Scholar] [CrossRef]
- Hou, M.; Andersson, M.; Eksborg, S.; Soder, O.; Jahnukainen, K. Xenotransplantation of testicular tissue into nude mice can be used for detecting leukemic cell contamination. Hum. Reprod. 2007, 22, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- De Michele, F.; Vermeulen, M.; Wyns, C. Fertility restoration with spermatogonial stem cells. Curr. Opin. Endocrinol. Diabetes Obes. 2017. [Google Scholar] [CrossRef] [PubMed]
- De Michele, F.; Poels, J.; Weerens, L.; Petit, C.; Evrard, Z.; Ambroise, J.; Gruson, D.; Wyns, C. Preserved seminiferous tubule integrity with spermatogonial survival and induction of sertoli and leydig cell maturation after long-term organotypic culture of prepubertal human testicular tissue. Hum. Reprod. 2017, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; Van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef]
- Jadoul, P.; Guilmain, A.; Squifflet, J.; Luyckx, M.; Votino, R.; Wyns, C.; Dolmans, M.M. Efficacy of ovarian tissue cryopreservation for fertility preservation: Lessons learned from 545 cases. Hum. Reprod. 2017, 32, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in reproductive tissue engineering: A review on its application as a biomaterial for fertility preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef] [PubMed]
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsepelidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum. Reprod. 2015, 30, 2107–2109. [Google Scholar] [CrossRef] [PubMed]
- Van Eyck, A.S.; Jordan, B.F.; Gallez, B.; Heilier, J.F.; Van Langendonckt, A.; Donnez, J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil. Steril. 2009, 92, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.M.; Martinez-Madrid, B.; Gadisseux, E.; Guiot, Y.; Yuan, W.Y.; Torre, A.; Camboni, A.; Van Langendonckt, A.; Donnez, J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction 2007, 134, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Geens, M.; De Block, G.; Tournaye, H. Spermatogonial survival in long-term human prepubertal xenografts. Fertil. Steril. 2008, 90, 2019–2022. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Nozawa, S.; Yoshiike, M.; Arai, M.; Sasaki, C.; Iwamoto, T. Xenografting of testicular tissue from an infant human donor results in accelerated testicular maturation. Hum. Reprod. 2010, 25, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Van Saen, D.; Goossens, E.; Haentjens, P.; Baert, Y.; Tournaye, H. Exogenous administration of recombinant human fsh does not improve germ cell survival in human prepubertal xenografts. Reprod. Biomed. Online 2013, 26, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Poels, J.; Van Langendonckt, A.; Many, M.C.; Wese, F.X.; Wyns, C. Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod. 2013, 28, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Poels, J.; Abou-Ghannam, G.; Herman, S.; Van Langendonckt, A.; Wese, F.X.; Wyns, C. In search of better spermatogonial preservation by supplementation of cryopreserved human immature testicular tissue xenografts with n-acetylcysteine and testosterone. Front. Surg. 2014, 1, 47. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Honaramooz, A.; Ehmcke, J.; Goebell, P.J.; Rubben, H.; Dhir, R.; Dobrinski, I.; Patrizio, P. Limited survival of adult human testicular tissue as ectopic xenograft. Hum. Reprod. 2006, 21, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, L.E.; Hemo, I.; Keshet, E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by pdgf-b and vegf. Development 1998, 125, 1591–1598. [Google Scholar] [PubMed]
- Geens, M.; De Block, G.; Goossens, E.; Frederickx, V.; Van Steirteghem, A.; Tournaye, H. Spermatogonial survival after grafting human testicular tissue to immunodeficient mice. Hum. Reprod. 2006, 21, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Van Saen, D.; Goossens, E.; Bourgain, C.; Ferster, A.; Tournaye, H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum. Reprod. 2011, 26, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Kulin, H.E.; Frontera, M.A.; Demers, L.M.; Bartholomew, M.J.; Lloyd, T.A. The onset of sperm production in pubertal boys. Relationship to gonadotropin excretion. Am. J. Dis. Child. 1989, 143, 190–193. [Google Scholar] [PubMed]
- Honaramooz, A.; Snedaker, A.; Boiani, M.; Scholer, H.; Dobrinski, I.; Schlatt, S. Sperm from neonatal mammalian testes grafted in mice. Nature 2002, 418, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Jahnukainen, K.; Ehmcke, J.; Nurmio, M.; Schlatt, S. Autologous ectopic grafting of cryopreserved testicular tissue preserves the fertility of prepubescent monkeys that receive sterilizing cytotoxic therapy. Cancer Res. 2012, 72, 5174–5178. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Inoue, K.; Ogonuki, N.; Kanatsu-Shinohara, M.; Miki, H.; Nakata, K.; Kurome, M.; Nagashima, H.; Toyokuni, S.; Kogishi, K.; et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in vitro microinsemination. Hum. Reprod. 2002, 17, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Snedaker, A.K.; Honaramooz, A.; Dobrinski, I. A game of cat and mouse: Xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J. Androl. 2004, 25, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Anzar, M.; Yang, Y.; Honaramooz, A. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology 2010, 73, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, C.; Mullen, B.; Jarvi, K.; McKerlie, C.; Lo, K.C. Effect of different cryoprotectant agents on spermatogenesis efficiency in cryopreserved and grafted neonatal mouse testicular tissue. Cryobiology 2013, 67, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Pukazhenthi, B.S.; Nagashima, J.; Travis, A.J.; Costa, G.M.; Escobar, E.N.; Franca, L.R.; Wildt, D.E. Slow freezing, but not vitrification supports complete spermatogenesis in cryopreserved, neonatal sheep testicular xenografts. PLoS ONE 2015, 10, e0123957. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cheng, K.M.; Silversides, F.G. Production of live offspring from testicular tissue cryopreserved by vitrification procedures in japanese quail (coturnix japonica). Biol. Reprod. 2013, 88, 124. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Wakayama, T. Generation of normal progeny by intracytoplasmic sperm injection following grafting of testicular tissue from cloned mice that died postnatally. Biol. Reprod. 2005, 73, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Kikuchi, K.; Nakai, M.; Somfai, T.; Noguchi, J.; Tanihara, F.; Ito, J.; Kashiwazaki, N. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS ONE 2013, 8, e70989. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Nie, Y.H.; Zhang, C.C.; Cai, Y.J.; Wang, Y.; Lu, H.P.; Li, Y.Z.; Cheng, C.; Qiu, Z.L.; Sun, Q. Generation of macaques with sperm derived from juvenile monkey testicular xenografts. Cell Res. 2016, 26, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Poels, J.; de Michele, F.; des Rieux, A.; Wyns, C. Restoring fertility with cryopreserved prepubertal testicular tissue: Perspectives with hydrogel encapsulation, nanotechnology, and bioengineered scaffolds. Ann. Biomed. Eng. 2017. [Google Scholar] [CrossRef]
- Schlatt, S.; Kim, S.S.; Gosden, R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and heterotopic grafting to castrated hosts. Reproduction 2002, 124, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Arregui, L.; Dobrinski, I. Xenografting of testicular tissue pieces: 12 years of an in vivo spermatogenesis system. Reproduction 2014, 148, R71–R84. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Westernstroer, B.; Gassei, K.; Ehmcke, J. Donor-host involvement in immature rat testis xenografting into nude mouse hosts. Biol. Reprod. 2010, 82, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Andrejecsk, J.W.; Cui, J.; Chang, W.G.; Devalliere, J.; Pober, J.S.; Saltzman, W.M. Paracrine exchanges of molecular signals between alginate-encapsulated pericytes and freely suspended endothelial cells within a 3d protein gel. Biomaterials 2013, 34, 8899–8908. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.; Allison, J.E.; Stanley, A.J.; Gumbreck, L.G. Reciprocal transplantation of testes between normal and pseudohermaphroditic male rats. Fertil. Steril. 1969, 20, 482–494. [Google Scholar] [CrossRef]
- Johnson, L.; Suggs, L.C.; Norton, Y.M.; Zeh, W.C. Effect of developmental age or time after transplantation on sertoli cell number and testicular size in inbred fischer rats. Biol. Reprod. 1996, 54, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Wistuba, J.; Luetjens, C.M.; Wesselmann, R.; Nieschlag, E.; Simoni, M.; Schlatt, S. Meiosis in autologous ectopic transplants of immature testicular tissue grafted to callithrix jacchus. Biol. Reprod. 2006, 74, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Luetjens, C.M.; Stukenborg, J.B.; Nieschlag, E.; Simoni, M.; Wistuba, J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology 2008, 149, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Van Saen, D.; Goossens, E.; De Block, G.; Tournaye, H. Regeneration of spermatogenesis by grafting testicular tissue or injecting testicular cells into the testes of sterile mice: A comparative study. Fertil. Steril. 2009, 91, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Reeves, J.J.; McLean, D.J. Establishment of spermatogenesis in neonatal bovine testicular tissue following ectopic xenografting varies with donor age. Biol. Reprod. 2005, 72, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; de Avila, J.M.; McLean, D.J. Grafting period and donor age affect the potential for spermatogenesis in bovine ectopic testis xenografts. Biol. Reprod. 2006, 75, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Jahnukainen, K.; Ehmcke, J.; Hergenrother, S.D.; Schlatt, S. Effect of cold storage and cryopreservation of immature non-human primate testicular tissue on spermatogonial stem cell potential in xenografts. Hum. Reprod. 2007, 22, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.J.; DeFalco, T. Role of the testis interstitial compartment in spermatogonial stem cell function. Reproduction 2017, 153, R151–R162. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Honaramooz, A.; Boiani, M.; Scholer, H.R.; Dobrinski, I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol. Reprod. 2003, 68, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Honaramooz, A.; Li, M.W.; Penedo, M.C.; Meyers, S.; Dobrinski, I. Accelerated maturation of primate testis by xenografting into mice. Biol. Reprod. 2004, 70, 1500–1503. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Gassei, K.; Westernstroer, B.; Ehmcke, J. Modulating testicular mass in xenografting: A model to explore testis development and endocrine function. Endocrinology 2010, 151, 4018–4023. [Google Scholar] [CrossRef] [PubMed]
- Wistuba, J.; Mundry, M.; Luetjens, C.M.; Schlatt, S. Cografting of hamster (phodopus sungorus) and marmoset (callithrix jacchus) testicular tissues into nude mice does not overcome blockade of early spermatogenic differentiation in primate grafts. Biol. Reprod. 2004, 71, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Rathi, R.; Zeng, W.; Megee, S.; Conley, A.; Meyers, S.; Dobrinski, I. Maturation of testicular tissue from infant monkeys after xenografting into mice. Endocrinology 2008, 149, 5288–5296. [Google Scholar] [CrossRef] [PubMed]
- Ehmcke, J.; Gassei, K.; Westernstroer, B.; Schlatt, S. Immature rhesus monkey (macaca mulatta) testis xenografts show increased growth, but not enhanced seminiferous differentiation, under human chorionic gonadotropin treatment of nude mouse recipients. Int. J. Androl. 2011, 34, e459–e467. [Google Scholar] [CrossRef] [PubMed]
- Rathi, R.; Honaramooz, A.; Zeng, W.; Turner, R.; Dobrinski, I. Germ cell development in equine testis tissue xenografted into mice. Reproduction 2006, 131, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Marchlewska, K.; Kula, K.; Walczak-Jedrzejowska, R.; Kula, W.; Oszukowska, E.; Filipiak, E.; Moszura, T.; Slowikowska-Hilczer, J. Maturational changes in connexin 43 expression in the seminiferous tubules may depend on thyroid hormone action. Arch. Med. Sci. 2013, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Sosa, J.R.; Costa, G.M.; Rathi, R.; Franca, L.R.; Dobrinski, I. Endocrine modulation of the recipient environment affects development of bovine testis tissue ectopically grafted in mice. Reproduction 2012, 144, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.T.; Tung, K.S.; Tomomasa, H.; Wilson, L.W. Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol. Reprod. 1997, 57, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2011, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Caires, K.C.; de Avila, J.M.; Cupp, A.S.; McLean, D.J. Vegfa family isoforms regulate spermatogonial stem cell homeostasis in vivo. Endocrinology 2012, 153, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Ergun, S.; Kilic, N.; Fiedler, W.; Mukhopadhyay, A.K. Vascular endothelial growth factor and its receptors in normal human testicular tissue. Mol. Cell. Endocrinol. 1997, 131, 9–20. [Google Scholar] [CrossRef]
- Tian, R.; Yang, S.; Zhu, Y.; Zou, S.; Li, P.; Wang, J.; Zhu, Z.; Huang, Y.; He, Z.; Li, Z. Vegf/vegfr2 signaling regulates germ cell proliferation in vitro and promotes mouse testicular regeneration in vivo. Cells Tissues Organs 2016, 201, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; de Avila, J.M.; McLean, D.J. Effect of vascular endothelial growth factor and testis tissue culture on spermatogenesis in bovine ectopic testis tissue xenografts. Biol. Reprod. 2006, 75, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Caires, K.C.; de Avila, J.; McLean, D.J. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction 2009, 138, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Zafarullah, M.; Li, W.Q.; Sylvester, J.; Ahmad, M. Molecular mechanisms of n-acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Cay, A.; Alver, A.; Kucuk, M.; Isik, O.; Eminagaoglu, M.S.; Karahan, S.C.; Deger, O. The effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsion. J. Surg. Res. 2006, 131, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, S.; Mentese, A.; Karaguzel, E.; Karaca, Y.; Kucuk, A.; Uzun, A.; Yulug, E.; Turedi, S. A comparison of the effects of n-acetylcysteine and ethyl pyruvate on experimental testicular ischemia-reperfusion injury. Fertil. Steril. 2012, 98, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Erkkila, K.; Hirvonen, V.; Wuokko, E.; Parvinen, M.; Dunkel, L. N-acetyl-l-cysteine inhibits apoptosis in human male germ cells in vitro. J. Clin. Endocrinol. Metab. 1998, 83, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Reiter, R.J.; Hardeland, R.; Tan, D.X.; Barlow-Walden, L.R. Melatonin and structurally-related, endogenous indoles act as potent electron donors and radical scavengers in vitro. Redox Rep. 1996, 2, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Saki, G.; Hemadi, M.; Khodadadi, A.; Mohammadi-Asl, J. Melatonin improves spermatogonial stem cells transplantation efficiency in azoospermic mice. Iran. J. Basic Med. Sci. 2014, 17, 93–99. [Google Scholar] [PubMed]
- Navid, S.; Rastegar, T.; Baazm, M.; Alizadeh, R.; Talebi, A.; Gholami, K.; Khosravi-Farsani, S.; Koruji, M.; Abbasi, M. In vitro effects of melatonin on colonization of neonate mouse spermatogonial stem cells. Syst. Biol. Reprod. Med. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gholami, M.; Saki, G.; Hemadi, M.; Khodadadi, A.; Mohamma-di-Asl, J. Effect of melatonin on the expression of apoptotic genes in vitrified-thawed spermatogonia stem cells type a of 6-day-old mice. Iran. J. Basic Med. Sci. 2013, 16, 906–909. [Google Scholar] [PubMed]
- Kimsa, M.C.; Strzalka-Mrozik, B.; Kimsa, M.W.; Gola, J.; Nicholson, P.; Lopata, K.; Mazurek, U. Porcine endogenous retroviruses in xenotransplantation—Molecular aspects. Viruses 2014, 6, 2062–2083. [Google Scholar] [CrossRef] [PubMed]
- Nomi, M.A.A.; Coppi, P.D.; Soker, S. Principals of neovascularization for tissue engineering. Mol. Asp. Med. 2002, 23, 463–483. [Google Scholar] [CrossRef]
- Vajanto, I.; Rissanen, T.T.; Rutanen, J.; Hiltunen, M.O.; Tuomisto, T.T.; Arve, K.; Narvanen, O.; Manninen, H.; Rasanen, H.; Hippelainen, M.; et al. Evaluation of angiogenesis and side effects in ischemic rabbit hindlimbs after intramuscular injection of adenoviral vectors encoding vegf and lacz. J. Gene Med. 2002, 4, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Moulisova, V.; Gonzalez-Garcia, C.; Cantini, M.; Rodrigo-Navarro, A.; Weaver, J.; Costell, M.; Sabater, I.S.R.; Dalby, M.J.; Garcia, A.J.; Salmeron-Sanchez, M. Engineered microenvironments for synergistic vegf—Integrin signalling during vascularization. Biomaterials 2017, 126, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Brinster, R.L.; Zimmermann, J.W. Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. USA 1994, 91, 11298–11302. [Google Scholar] [CrossRef] [PubMed]
- Brook, P.F.; Radford, J.A.; Shalet, S.M.; Joyce, A.D.; Gosden, R.G. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil. Steril. 2001, 75, 269–274. [Google Scholar] [CrossRef]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Inoue, K.; Ogura, A.; Toyokuni, S.; Shinohara, T. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Hum. Reprod. 2003, 18, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Schlatt, S.; Rosiepen, G.; Weinbauer, G.F.; Rolf, C.; Brook, P.F.; Nieschlag, E. Germ cell transfer into rat, bovine, monkey and human testes. Hum. Reprod. 1999, 14, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Meng, J.; Goossens, E.; Lahoutte, T.; Marichal, M.; Tournaye, H. In search of an efficient injection technique for future clinical application of spermatogonial stem cell transplantation: Infusion of contrast dyes in isolated cadaveric human testes. Fertil. Steril. 2012, 98, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, D.E.; Avarbock, M.R.; Maika, S.D.; Hammer, R.E.; Brinster, R.L. Rat spermatogenesis in mouse testis. Nature 1996, 381, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Dobrinski, I.; Avarbock, M.R.; Brinster, R.L. Xenogeneic spermatogenesis following transplantation of hamster germ cells to mouse testes. Biol. Reprod. 1999, 60, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.D.; Brinster, R.L. Ultrastructural observations of spermatogenesis following transplantation of rat testis cells into mouse seminiferous tubules. J. Androl. 1996, 17, 615–627. [Google Scholar] [PubMed]
- Nagano, M.; Patrizio, P.; Brinster, R.L. Long-term survival of human spermatogonial stem cells in mouse testes. Fertil. Steril. 2002, 78, 1225–1233. [Google Scholar] [CrossRef]
- Wu, X.; Schmidt, J.A.; Avarbock, M.R.; Tobias, J.W.; Carlson, C.A.; Kolon, T.F.; Ginsberg, J.P.; Brinster, R.L. Prepubertal human spermatogonia and mouse gonocytes share conserved gene expression of germline stem cell regulatory molecules. Proc. Natl. Acad. Sci. USA 2009, 106, 21672–21677. [Google Scholar] [CrossRef] [PubMed]
- Sadri-Ardekani, H.; Akhondi, M.A.; van der Veen, F.; Repping, S.; van Pelt, A.M. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA 2011, 305, 2416–2418. [Google Scholar] [CrossRef] [PubMed]
- Radford, J.; Shalet, S.; Lieberman, B. Fertility after treatment for cancer. Questions remain over ways of preserving ovarian and testicular tissue. BMJ 1999, 319, 935–936. [Google Scholar] [CrossRef] [PubMed]
- Radford, J. Restoration of fertility after treatment for cancer. Horm. Res. 2003, 59 (Suppl. S1), 21–23. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Skakkebaek, N.E. Quantification of germ cells and seminiferous tubules by stereological examination of testicles from 50 boys who suffered from sudden death. Int. J. Androl. 1983, 6, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Dobrinski, I.; Ogawa, T.; Avarbock, M.R.; Brinster, R.L. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol. Reprod. Dev. 1999, 53, 142–148. [Google Scholar] [CrossRef]
- Sadri-Ardekani, H.; Mizrak, S.C.; van Daalen, S.K.; Korver, C.M.; Roepers-Gajadien, H.L.; Koruji, M.; Hovingh, S.; de Reijke, T.M.; de la Rosette, J.J.; van der Veen, F.; et al. Propagation of human spermatogonial stem cells in vitro. JAMA 2009, 302, 2127–2134. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour, T.; Movahedin, M.; Koruji, M.; Nowroozi, M.R. Xenotransplantation assessment: Morphometric study of human spermatogonial stem cells in recipient mouse testes. Andrologia 2015, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Conrad, S.; Azizi, H.; Hatami, M.; Kubista, M.; Bonin, M.; Hennenlotter, J.; Renninger, M.; Skutella, T. Differential gene expression profiling of enriched human spermatogonia after short- and long-term culture. Biomed. Res. Int. 2014, 2014, 138350. [Google Scholar] [CrossRef] [PubMed]
- Gat, I.; Maghen, L.; Filice, M.; Kenigsberg, S.; Wyse, B.; Zohni, K.; Saraz, P.; Fisher, A.G.; Librach, C. Initial germ cell to somatic cell ratio impacts the efficiency of ssc expansion in vitro. Syst. Biol. Reprod. Med. 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Arauzo-Bravo, M.J.; Tapia, N.; Kim, J.; Lin, Q.; Bernemann, C.; Han, D.W.; Gentile, L.; Reinhardt, P.; Greber, B.; et al. Human adult germline stem cells in question. Nature 2010, 465, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shira Maymon, B.; Yogev, L.; Marks, A.; Hauser, R.; Botchan, A.; Yavetz, H. Sertoli cell inactivation by cytotoxic damage to the human testis after cancer chemotherapy. Fertil. Steril. 2004, 81, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.J.; Radford, J.A.; Ryder, W.D.; Shalet, S.M. Testicular function after cytotoxic chemotherapy: Evidence of leydig cell insufficiency. J. Clin. Oncol. 1999, 17, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Ohta, H.; Tsujimura, A.; Takao, T.; Miyagawa, Y.; Takada, S.; Matsumiya, K.; Wakayama, T.; Okuyama, A. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J. Clin. Investig. 2005, 115, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Tsujimura, A.; Miyagawa, Y.; Kiuchi, H.; Matsuoka, Y.; Takao, T.; Takada, S.; Nonomura, N.; Okuyama, A. Isolation of germ cells from leukemia and lymphoma cells in a human in vitro model: Potential clinical application for restoring human fertility after anticancer therapy. Cancer Res. 2006, 66, 11166–11171. [Google Scholar] [CrossRef] [PubMed]
- Geens, M.; Van de Velde, H.; De Block, G.; Goossens, E.; Van Steirteghem, A.; Tournaye, H. The efficiency of magnetic-activated cell sorting and fluorescence-activated cell sorting in the decontamination of testicular cell suspensions in cancer patients. Hum. Reprod. 2007, 22, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Dovey, S.L.; Valli, H.; Hermann, B.P.; Sukhwani, M.; Donohue, J.; Castro, C.A.; Chu, T.; Sanfilippo, J.S.; Orwig, K.E. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J. Clin. Investig. 2013, 123, 1833–1843. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Andersson, M.; Zheng, C.; Sundblad, A.; Soder, O.; Jahnukainen, K. Decontamination of leukemic cells and enrichment of germ cells from testicular samples from rats with roser’s t-cell leukemia by flow cytometric sorting. Reproduction 2007, 134, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Hermann, B.P.; Sukhwani, M.; Salati, J.; Sheng, Y.; Chu, T.; Orwig, K.E. Separating spermatogonia from cancer cells in contaminated prepubertal primate testis cell suspensions. Hum. Reprod. 2011, 26, 3222–3231. [Google Scholar] [CrossRef] [PubMed]
- Sadri-Ardekani, H.; Homburg, C.H.; van Capel, T.M.; van den Berg, H.; van der Veen, F.; van der Schoot, C.E.; van Pelt, A.M.; Repping, S. Eliminating acute lymphoblastic leukemia cells from human testicular cell cultures: A pilot study. Fertil. Steril. 2014, 101, 1072–1078. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Ogonuki, N.; Iwano, T.; Lee, J.; Kazuki, Y.; Inoue, K.; Miki, H.; Takehashi, M.; Toyokuni, S.; Shinkai, Y.; et al. Genetic and epigenetic properties of mouse male germline stem cells during long-term culture. Development 2005, 132, 4155–4163. [Google Scholar] [CrossRef] [PubMed]
- Nickkholgh, B.; Mizrak, S.C.; van Daalen, S.K.; Korver, C.M.; Sadri-Ardekani, H.; Repping, S.; van Pelt, A.M. Genetic and epigenetic stability of human spermatogonial stem cells during long-term culture. Fertil. Steril. 2014, 102, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, S.; Feiner, R.; Dvir, T. Cardiac tissue engineering: From matrix design to the engineering of bionic hearts. Regen. Med. 2017, 12, 275–284. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–96. [Google Scholar]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Tekade, M.; Kesharwani, P.; Iyer, A.K.; Kalia, K.; Tekade, R.K. The use of nanoscaffolds and dendrimers in tissue engineering. Drug Discov. Today 2017, 22, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Kaproth, M.T.; Parks, J.E. In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol. Reprod. 2001, 65, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Janson, I.A.; Putnam, A.J. Extracellular matrix elasticity and topography: Material-based cues that affect cell function via conserved mechanisms. J. Biomed. Mater. Res. A 2015, 103, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Qazi, T.H.; Mooney, D.J.; Duda, G.N.; Geissler, S. Biomaterials that promote cell-cell interactions enhance the paracrine function of mscs. Biomaterials 2017, 140, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Chang, C.C.; Zhang, Z.; Li, Q. Characterization of tissue scaffolds for time-dependent biotransport criteria—A novel computational procedure. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Wong, E.W.; Yan, H.H.; Mruk, D.D. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: New insights and advances. Mol. Cell. Endocrinol. 2010, 315, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pollanen, P.P.; Kallajoki, M.; Risteli, L.; Risteli, J.; Suominen, J.J. Laminin and type iv collagen in the human testis. Int. J. Androl. 1985, 8, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.H.; Stamer, D.K.; Kyriakides, T.R. The host response to naturally-derived extracellular matrix biomaterials. Semin. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, S.; Li, Q. Microstructure design of biodegradable scaffold and its effect on tissue regeneration. Biomaterials 2011, 32, 5003–5014. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Ding, J. Poly(lactide-co-glycolide) porous scaffolds for tissue engineering and regenerative medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, J.; Jing, D.; Ding, J. “Wet-state” mechanical properties of three-dimensional polyester porous scaffolds. J. Biomed. Mater. Res. A 2006, 76, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Poels, J.; Abou-Ghannam, G.; Decamps, A.; Leyman, M.; Rieux, A.D.; Wyns, C. Transplantation of testicular tissue in alginate hydrogel loaded with vegf nanoparticles improves spermatogonial recovery. J. Control. Release 2016, 234, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17 (Suppl. S4), 467–479. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Liu, W.; Yang, L. A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine. J. Biomed. Mater. Res. A 2017, 105, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Dorkoosh, F.A.; Dehpour, A.R.; Moezi, L.; Larijani, B.; Junginger, H.E.; Rafiee-Tehrani, M. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: Ex vivo and in vivo studies. Int. J. Pharm. 2008, 356, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Vitharana, S.N.; Peek, L.J.; Coop, T.; Berkland, C. Polyelectrolyte complexes stabilize and controllably release vascular endothelial growth factor. Biomacromolecules 2007, 8, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.S.; Cho, C.W. A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells. Oncotarget 2017, 8, 30369–30382. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, M.; Kelly, M.E.; Chen, X. Regulation of sequential release of growth factors using bilayer polymeric nanoparticles for cardiac tissue engineering. Nanomedicine 2016, 11, 3237–3259. [Google Scholar] [CrossRef] [PubMed]
- Najjar, M.; Manzoli, V.; Abreu, M.; Villa, C.; Martino, M.M.; Molano, R.D.; Torrente, Y.; Pileggi, A.; Inverardi, L.; Ricordi, C.; et al. Fibrin gels engineered with pro-angiogenic growth factors promote engraftment of pancreatic islets in extrahepatic sites in mice. Biotechnol. Bioeng. 2015, 112, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Felice, B.; Prabhakaran, M.P.; Rodriguez, A.P.; Ramakrishna, S. Drug delivery vehicles on a nano-engineering perspective. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gye, M.C.; Choi, K.W.; Hong, J.Y.; Lee, Y.B.; Park, D.W.; Lee, S.J.; Min, C.K. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 2007, 87, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Reuter, K.; Ehmcke, J.; Stukenborg, J.B.; Simoni, M.; Damm, O.S.; Redmann, K.; Schlatt, S.; Wistuba, J. Reassembly of somatic cells and testicular organogenesis in vitro. Tissue Cell 2014, 46, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Jalayeri, M.; Pirnia, A.; Najafabad, E.P.; Varzi, A.M.; Gholami, M. Evaluation of alginate hydrogel cytotoxicity on three-dimensional culture of type a spermatogonial stem cells. Int. J. Biol. Macromol. 2017, 95, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Artel, A.; Mehdizadeh, H.; Chiu, Y.C.; Brey, E.M.; Cinar, A. An agent-based model for the investigation of neovascularization within porous scaffolds. Tissue Eng. Part A 2011, 17, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Jiang, X.; Jiang, Y.; Hu, X.; Mou, H.; Li, M.; Guan, H. In vitro antioxidative activities of three marine oligosaccharides. Nat. Prod. Res. 2007, 21, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.H.; Ji, A.T.; Chang, C.C.; Cheng, C.J.; Lee, L.M.; Ho, J.H. Local injection of mesenchymal stem cells protects testicular torsion-induced germ cell injury. Stem Cell Res. Ther. 2015, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Horibe, A.; Eid, N.; Ito, Y.; Hamaoka, H.; Tanaka, Y.; Kondo, Y. Upregulated autophagy in sertoli cells of ethanol-treated rats is associated with induction of inducible nitric oxide synthase (inos), androgen receptor suppression and germ cell apoptosis. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef]
- Eid, N.; Kondo, Y. Ethanol-induced mitophagy in rat sertoli cells: Implications for male fertility. Andrologia 2017. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Stukenborg, J.B.; Landreh, M.; De Kock, J.; Jornvall, H.; Soder, O.; Goossens, E. Derivation and characterization of a cytocompatible scaffold from human testis. Hum. Reprod. 2015, 30, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Del Vento, F.; de Michele, F.; Poels, J.; Wyns, C. Development of a cytocompatible scaffold from pig immature testicular tissue allowing human sertoli cell attachment, proliferation and functionality. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Rombaut, C.; Goossens, E. Scaffold-based and scaffold-free testicular organoids from primary human testicular cells. Methods Mol. Biol. 2017. [Google Scholar] [CrossRef]
- Wang, W.; Mo, Z.; Ye, B.; Hu, P.; Liu, S.; Yi, S. A clinical trial of xenotransplantation of neonatal pig islets for diabetic patients. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2011, 36, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Ciubotaru, A.; Cebotari, S.; Tudorache, I.; Beckmann, E.; Hilfiker, A.; Haverich, A. Biological heart valves. Biomed. Tech. 2013, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikura, Y.; Yabuta, Y.; Ohta, H.; Hayashi, K.; Nakamura, T.; Okamoto, I.; Yamamoto, T.; Kurimoto, K.; Shirane, K.; Sasaki, H.; et al. In vitro derivation and propagation of spermatogonial stem cell activity from mouse pluripotent stem cells. Cell Rep. 2016, 17, 2789–2804. [Google Scholar] [CrossRef] [PubMed]
- Chuma, S.; Kanatsu-Shinohara, M.; Inoue, K.; Ogonuki, N.; Miki, H.; Toyokuni, S.; Hosokawa, M.; Nakatsuji, N.; Ogura, A.; Shinohara, T. Spermatogenesis from epiblast and primordial germ cells following transplantation into postnatal mouse testis. Development 2005, 132, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust in vitro induction of human germ cell fate from pluripotent stem cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Botman, O.; Wyns, C. Induced pluripotent stem cell potential in medicine, specifically focused on reproductive medicine. Front. Surg. 2014, 1, 5. [Google Scholar] [CrossRef] [PubMed]


| Reference | Donor | Grafting Site | Graft Size | Cryopreserved or Fresh | Castrated Host | Outcome |
|---|---|---|---|---|---|---|
| Wyns et al., 2007; [12] | Prepubertal | Peritoneum scrotal bursa | 2–9 mm3 | F/T | yes | SG survival after 3 weeks: 14 % |
| Wyns et al., 2008; [16] | Prepubertal | Peritoneum scrotal bursa | 2–8 mm3 | F/T | yes | SG survival 3.7%, numerous premeiotic spermatocytes, a few spermatocytes at the pachytene stage and spermatid and spermatozoa-like cells, without expression of the meiotic and post-meiotic markers |
| Goossens et al., 2008; [27] | Prepubertal 10 and 11 y.o. | Back skin | 2–10 mm3 | fresh | no | Some rare SG survival after 4–9 months |
| Sato et al., 2010; [28] | 3 y.o. testicular hemangioma | Back skin | 0.5–1 mm3 | fresh | yes | Pachytene spermatocytes after one year |
| Van Saen et al., 2013; [29] | Prepubertal | Intra- testicular | 1.5–3 mm3 | Fresh and F/T | No | No effect of FSH administration or slow-freezing primary pachytene spermatocytes 9 and 12 months after grafting |
| Poels et al., 2013; [30] | Prepubertal | Peritoneum scrotal bursea | 1 mm3 | Fresh, slow-frozen and vitrified | yes | SG survival after 6 months: 3.4%, 4.1%, and 7.3%, respectively, for fresh, slow-frozen-thawed and vitrified-warmed tissue. No statistical significant difference between three groups. |
| Poels et al 2014; [31] | Prepubertal | Peritoneum scrotal bursa | 1 mm3 | F/T | yes | SG survival after 5 days, 67%, 63%, and 53%, respectively, for slow-frozen tissue, slow-frozen tissue supplemented with NAC, and slow-frozen tissue supplemented with FSH and testosterone. No impact of NAC or FSH/Testosterone supplementation on SG survival |
| Schlatt et al., 2006; [32] | Adult4 Azoospermic patients, 1 Cancer survivor, 1 Testicular cancer patient, 3 transsexual patients. | Back skin | 0.5–1 mm3 | fresh | yes | Occasional Type A SG survival No correction of spermatogenesis disruption after xenografting |
| Geens et al., 2006; [34] | Adult | Back skin | 4 mm3 | fresh | yes | SG survival 33% < 120 d 14% > 120 d |
| Van Saen et al., 2011; [35] | Prepubertal POST-CHEMOTHERAPY Postpubertal (12 and 13 y.o.) | Intra-testicular | 6 mm3 | F/T | no | 4 months: SG survival 0,2/ST. 9 months: SG survival 0,6/ST. Higher SG survival in xenografts from the postpubertal donors. No SG differentiation in younger patients ‘tissue, two older donors’ tissue with differentiation up to primary spermatocyte and secondary spermatocytes in the oldest donor after 9 months. |
| Reference | Species | Technique | Outcome (Residual Contamination/Contamination of Samples or Contamination of Mice after Transplantation) |
|---|---|---|---|
| Fujita et al., 2005; [116] | Mouse | FACS | No contamination of recipient mice |
| Fujita et al., 2006; [117] | Human | FACS | Malignant cells in 1/8 in vitro cultures |
| Geens et al., 2007; [118] | Mouse | MACS + FACS | Malignant cells in 1/32 in vitro cultures 43% of mice contaminated after transplantation |
| Human | FACS | 10/11 contaminated cultures | |
| Dovey et al., 2013; [119] | Human | FACS | Post FACS Purity check was only 98.8–99.9% No tumour formation after xenotransplantation of sorted cell suspension to 55 nude mice (but tumour formation after contaminated cell transplantation was only 23–55%) |
| Hou et al., 2007; [120] | Rat | FACS | Germ cells selection or leukaemia cells isolation: contamination of 2/3 and 2/2 recipient rats Germ cell selection and leukaemia cells isolation: survival of all recipient rats |
| Hermann et al., 2011; [121] | Non-human primates | FACS | No tumour after nude mouse transplantation in 3 of 4 cell colonies |
| Sadri-Aderkani et al., 2014; [122] | Human | In vitro culture | Acute lymphoblastic leukaemia cells undetectable after 26 d |
| Matrix Mechanical Characteristics that Impact Cell or Tissue Function |
|---|
| • Pore size and morphology (Chan et al., 2008); [142] |
| • Elasticity (Janson et al., 2015); [131] |
| • Stiffness (Xia et al., 2017); [143] |
| • Hydration degree (Wu et al., 2006); [140] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Vento, F.; Vermeulen, M.; De Michele, F.; Giudice, M.G.; Poels, J.; Des Rieux, A.; Wyns, C. Tissue Engineering to Improve Immature Testicular Tissue and Cell Transplantation Outcomes: One Step Closer to Fertility Restoration for Prepubertal Boys Exposed to Gonadotoxic Treatments. Int. J. Mol. Sci. 2018, 19, 286. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010286
Del Vento F, Vermeulen M, De Michele F, Giudice MG, Poels J, Des Rieux A, Wyns C. Tissue Engineering to Improve Immature Testicular Tissue and Cell Transplantation Outcomes: One Step Closer to Fertility Restoration for Prepubertal Boys Exposed to Gonadotoxic Treatments. International Journal of Molecular Sciences. 2018; 19(1):286. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010286
Chicago/Turabian StyleDel Vento, Federico, Maxime Vermeulen, Francesca De Michele, Maria Grazia Giudice, Jonathan Poels, Anne Des Rieux, and Christine Wyns. 2018. "Tissue Engineering to Improve Immature Testicular Tissue and Cell Transplantation Outcomes: One Step Closer to Fertility Restoration for Prepubertal Boys Exposed to Gonadotoxic Treatments" International Journal of Molecular Sciences 19, no. 1: 286. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19010286