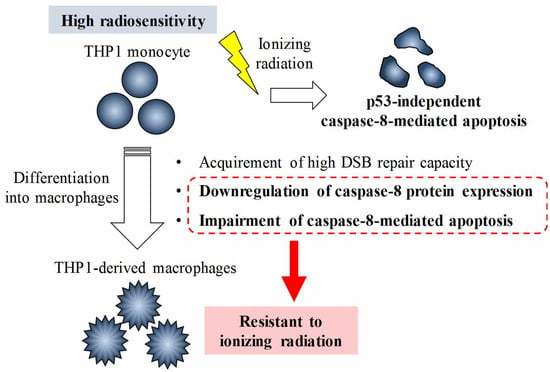
Relationship between the Regulation of Caspase-8-Mediated Apoptosis and Radioresistance in Human THP-1-Derived Macrophages
Abstract
:1. Introduction
2. Results
2.1. Effects of Ionizing Radiation on Apoptosis Induction in THP-1 Cells and THP-1-Derived Macrophages
2.2. Kinetics of γ-H2AX in THP-1 and Macrophages after X-Ray Irradiation
2.3. Effects of DSB Repair-Related Proteins Inhibitors on the Apoptosis Induction in Macrophages
2.4. Ionizing Radiation Induces Apoptosis in THP-1 Cells through the Caspase-8/Caspase-3 Pathway
2.5. Relationship between the Radioresistance of Macrophages and Caspase-8
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture and Treatment
4.3. In Vitro X-Ray Irradiation
4.4. Detection of Apoptotic Cells
4.5. Cell Death Analysis
4.6. Detection of γ-H2AX by Flow Cytometry
4.7. γ-H2AX Foci Analysis
4.8. Cell Cycle Analysis
4.9. Intracellular Phosphorylated-ATM Staining
4.10. SDS-PAGE and Western Blotting
4.11. Analysis of Cell Surface Fas Expression
4.12. qRT-PCR
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATM | Ataxia–telangiectasia mutated |
| ATR | Ataxia–telangiectasia and Rad-3-related |
| DMSO | Dimethyl sulfoxide |
| DNA-PKcs | DNA-dependent protein kinase, catalytic subunit |
| DSB | Double-strand breaks |
| ER | Endoplasmic reticulum |
| HR | Homologous recombination |
| IAP | Inhibitor of apoptosis protein |
| MFI | Mean fluorescence intensity |
| NHEJ | Non-homologous end joining |
| PARP | Poly ADP ribose polymerase |
| PI | Propidium iodide |
| PMA | Phorbol 12-myristate 13-acetate |
| TRAF2 | Tumor necrosis factor receptor-associated factor 2 |
| WB | Wash buffer |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- ICRP. 2012 ICRP Statement on Tissue Reactions/Early and Late Effects of Radiation in Normal Tissues and Organs—Threshold Doses for Tissue Reactions in a Radiation Protection Context; ICRP Publication 118. Ann. ICRP 41(1/2); ICRP: Ottawa, ON, Canada, 2012. [Google Scholar]
- Heylmann, D.; Rödel, F.; Kindler, T.; Kaina, B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta 2014, 1846, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, M.; Goldstein, M.; Heylmann, D.; Kaina, B. Human monocytes undergo excessive apoptosis following temozolomide activating the ATM/ATR pathway while dendritic cells and macrophages are resistant. PLoS ONE 2012, 7, e39956. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Goldstein, M.; Christmann, M.; Becker, H.; Heylmann, D.; Kaina, B. Human monocytes are severely impaired in base and DNA double-strand break repair that renders them vulnerable to oxidative stress. Proc. Natl. Acad. Sci. USA 2011, 108, 21105–21110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kumai, Y.; Kashiwakura, I. Effects of endoplasmic reticulum stress on apoptosis induction in radioresistant macrophages. Mol. Med. Rep. 2017, 15, 2867–2872. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Toyoshima, H.; Sakai, R.; Miyagawa, K.; Hagiwara, K.; Ishikawa, F.; Takaku, F.; Yazaki, Y.; Hirai, H. Frequent mutations in the p53 gene in human myeloid leukemia cell lines. Blood 1992, 79, 2378–2383. [Google Scholar] [PubMed]
- Hajra, K.M.; Liu, J.R. Apoptosome dysfunction in human cancer. Apoptosis 2004, 9, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Llambi, F. Cell death signaling. Cold Spring Harb. Perspect. Biol. 2015, 7, a006080. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Inanami, O.; Hayashi, M.; Kuwabara, M. Protein synthesis-dependent apoptotic signalling pathway in X-irradiated MOLT-4 human leukaemia cell line. Int. J. Radiat. Biol. 2002, 78, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, K.H.; Lee, S.J. Ionizing radiation utilizes c-Jun N-terminal kinase for amplification of mitochondrial apoptotic cell death in human cervical cancer cells. FEBS J. 2008, 275, 2096–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeggo, P.; Löbrich, M. Radiation-induced DNA damage responses. Radiat. Prot. Dosim. 2006, 122, 124–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandsma, I.; Gent, D.C. Pathway choice in DNA double strand break repair: Observations of a balancing act. Genome Integr. 2012, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakr, A.; Oing, C.; Köcher, S.; Borgmann, K.; Dornreiter, I.; Petersen, C.; Dikomey, E.; Mansour, W.Y. Involvement of ATM in homologous recombination after end resection and RAD51 nucleofilament formation. Nucleic Acids Res. 2015, 43, 3154–3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkaria, J.N.; Busby, E.C.; Tibbetts, R.S.; Roos, P.; Taya, Y.; Karnitz, L.M.; Abraham, R.T. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999, 59, 4375–4382. [Google Scholar] [PubMed]
- Fan, T.J.; Han, L.H.; Cong, R.S.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Hopkins-Donaldson, S.; Bodmer, J.L.; Bourloud, K.B.; Brognara, C.B.; Tschopp, J.; Gross, N. Loss of caspase-8 expression in highly malignant human neuroblastoma cells correlates with resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Cancer Res. 2000, 60, 4315–4319. [Google Scholar] [PubMed]
- Kim, P.K.; Mahidhara, R.; Seol, D.W. The role of caspase-8 in resistance to cancer chemotherapy. Drug Resist. Updates 2001, 4, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Afshar, G.; Jelluma, N.; Yang, X.; Basila, D.; Arvold, N.D.; Karlsson, A.; Yount, G.L.; Dansen, T.B.; Koller, E.; Haas-Kogan, D.A. Radiation-induced caspase-8 mediates p53-independent apoptosis in glioma cells. Cancer Res. 2006, 66, 4223–4232. [Google Scholar] [CrossRef] [PubMed]
- Tsurushima, H.; Yuan, X.; Dillehay, L.E.; Leong, K.W. Radiation-inducible caspase-8 gene therapy for malignant brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 517–525. [Google Scholar] [CrossRef] [PubMed]
- McCollum, G.; Keng, P.C.; States, J.C.; McCabe, M.J., Jr. Arsenite delays progression through each cell cycle phase and induces apoptosis following G2/M arrest in U937 myeloid leukemia cells. J. Pharmacol. Exp. Ther. 2005, 313, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, L.A.; Dupéré-Richer, D.; Pettersson, F.; Retrouvey, H.; Skoulikas, S.; Miller, W.H., Jr. Vorinostat induces reactive oxygen species and DNA damage in acute myeloid leukemia cells. PLoS ONE 2011, 6, e20987. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.H.; Chang, C.S.; Liu, H.H.; Tsai, Y.S.; Hsu, F.M.; Yu, Y.L.; Lai, C.K.; Gandee, L.; Pong, R.C.; Hsu, H.W.; et al. Sensitization of radio-resistant prostate cancer cells with a unique cytolethal distending toxin. Oncotarget 2014, 5, 5523–5534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Block, W.D.; Merkle, D.; Meek, K.; Lees-Miller, S.P. Selective inhibition of the DNA-dependent protein kinase (DNA-PK) by the radiosensitizing agent caffeine. Nucleic Acids Res. 2004, 32, 1967–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil del Alcazar, C.R.; Hardebeck, M.C.; Mukherjee, B.; Tomimatsu, N.; Gao, X.; Yan, J.; Xie, X.J.; Bachoo, R.; Li, L.; Habib, A.A.; et al. Inhibition of DNA double-strand break repair by the dual PI3K/mTOR inhibitor NVP-BEZ235 as a strategy for radiosensitization of glioblastoma. Clin. Cancer Res. 2014, 20, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Dolman, M.E.; van der Ploeg, I.; Koster, J.; Bate-Eya, L.T.; Versteeg, R.; Caron, H.N.; Molenaar, J.J. DNA-Dependent Protein Kinase As Molecular Target for Radiosensitization of Neuroblastoma Cells. PLoS ONE 2015, 10, e0145744. [Google Scholar] [CrossRef] [PubMed]
- So, E.Y.; Kozicki, M.; Ouchi, T. Roles of DNA Damage Response Proteins in Mitogen-Induced Thp-1 Differentiation into Macrophage. J. Cancer Biol. Res. 2013, 1, pii: 1004. [Google Scholar]
- Haupt, S.; Berger, M.; Goldberg, Z.; Haupt, Y. Apoptosis—The p53 network. J. Cell Sci. 2003, 116, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Little, J.B. p53 is involved in but not required for ionizing radiation-induced caspase-3 activation and apoptosis in human lymphoblast cell lines. Cancer Res. 1998, 58, 4277–4281. [Google Scholar] [PubMed]
- Sheikh, M.S.; Burns, T.F.; Huang, Y.; Wu, G.S.; Amundson, S.; Brooks, K.S.; Fornace, A.J., Jr.; el-Deiry, W.S. p53-dependent and -independent regulation of the death receptor KILLER/DR5 gene expression in response to genotoxic stress and tumor necrosis factor alpha. Cancer Res. 1998, 58, 1593–1598. [Google Scholar] [PubMed]
- Hamasu, T.; Inanami, O.; Asanuma, T.; Kuwabara, M. Enhanced induction of apoptosis by combined treatment of human carcinoma cells with X rays and death receptor agonists. J. Radiat. Res. 2005, 46, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kiener, P.A.; Davis, P.M.; Starling, G.C.; Mehlin, C.; Klebanoff, S.J.; Ledbetter, J.A.; Liles, W.C. Differential induction of apoptosis by Fas-Fas ligand interactions in human monocytes and macrophages. J. Exp. Med. 1997, 185, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.P.; Ban, K.; Dujka, M.E.; McConkey, D.J.; Munsell, M.; Palladino, M.; Chandra, J. NPI-0052, a novel proteasome inhibitor, induces caspase-8 and ROS-dependent apoptosis alone and in combination with HDAC inhibitors in leukemia cells. Blood 2007, 110, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.A.; Ullman, E.; Dou, Z.; Zong, W.X. Inhibition of protein degradation induces apoptosis through a microtubule-associated protein 1 light chain 3-mediated activation of caspase-8 at intracellular membranes. Mol. Cell. Biol. 2011, 31, 3158–3170. [Google Scholar] [CrossRef] [PubMed]
- Van Geelen, C.M.; Pennarun, B.; Ek, W.B.; Le, P.T.; Spierings, D.C.; De Vries, E.G.; De Jong, S. Downregulation of active caspase 8 as a mechanism of acquired TRAIL resistance in mismatch repair-proficient colon carcinoma cell lines. Int. J. Oncol. 2010, 37, 1031–1041. [Google Scholar] [PubMed]
- Fiandalo, M.V.; Schwarze, S.R.; Kyprianou, N. Proteasomal regulation of caspase-8 in cancer cell apoptosis. Apoptosis 2013, 18, 766–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalvez, F.; Lawrence, D.; Yang, B.; Yee, S.; Pitti, R.; Marsters, S.; Pham, V.C.; Stephan, J.P.; Lill, J.; Ashkenazi, A. TRAF2 Sets a threshold for extrinsic apoptosis by tagging caspase-8 with a ubiquitin shutoff timer. Mol. Cell 2012, 48, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Marivin, A.; Berthelet, J.; Plenchette, S.; Dubrez, L. The Inhibitor of Apoptosis (IAPs) in Adaptive Response to Cellular Stress. Cells 2012, 1, 711–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Fang, S.; Jensen, J.P.; Weissman, A.M.; Ashwell, J.D. Ubiquitin protein ligase activity of IAPs and their degradation in proteasomes in response to apoptotic stimuli. Science 2000, 288, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Chiba, K.; Saitoh, T.; Kashiwakura, I. Ionizing radiation affects the expression of Toll-like receptors 2 and 4 in human monocytic cells through c-Jun N-terminal kinase activation. J. Radiat. Res. 2014, 55, 876–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshino, H.; Iwabuchi, M.; Kazama, Y.; Furukawa, M.; Kashiwakura, I. Effects of retinoic acid-inducible gene-I-like receptors activations and ionizing radiation cotreatment on cytotoxicity against human non-small cell lung cancer in vitro. Oncol. Lett. 2018, 15, 4697–4705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakasaya, T.; Yoshino, H.; Fukushi, Y.; Yoshizawa, A.; Kashiwakura, I. A liquid crystal-related compound induces cell cycle arrest at the G2/M phase and apoptosis in the A549 human non-small cell lung cancer cell line. Int. J. Oncol. 2013, 42, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, S.; Yoshino, H.; Yoshizawa, A.; Kashiwakura, I. p53-independent structure-activity relationships of 3-ring mesogenic compounds’ activity as cytotoxic effects against human non-small cell lung cancer lines. BMC Cancer 2016, 16, 521. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Kashiwakura, I. Involvement of reactive oxygen species in ionizing radiation-induced upregulation of cell surface Toll-like receptor 2 and 4 expression in human monocytic cells. J. Radiat. Res. 2017, 58, 626–635. [Google Scholar] [CrossRef] [PubMed]






| Sequence (5′→3′) | |
|---|---|
| Caspase-8 F | CTTCCTGCCTGCCTGTACC |
| Caspase-8 R | CGTGCCCAGAAAGTGGAC |
| β-actin F | TGGCACCCAGCACAATGAA |
| β-actin R | CTAAGTCATAGTCCGCCTAGAAGCA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshino, H.; Konno, H.; Ogura, K.; Sato, Y.; Kashiwakura, I. Relationship between the Regulation of Caspase-8-Mediated Apoptosis and Radioresistance in Human THP-1-Derived Macrophages. Int. J. Mol. Sci. 2018, 19, 3154. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19103154
Yoshino H, Konno H, Ogura K, Sato Y, Kashiwakura I. Relationship between the Regulation of Caspase-8-Mediated Apoptosis and Radioresistance in Human THP-1-Derived Macrophages. International Journal of Molecular Sciences. 2018; 19(10):3154. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19103154
Chicago/Turabian StyleYoshino, Hironori, Haruka Konno, Koya Ogura, Yoshiaki Sato, and Ikuo Kashiwakura. 2018. "Relationship between the Regulation of Caspase-8-Mediated Apoptosis and Radioresistance in Human THP-1-Derived Macrophages" International Journal of Molecular Sciences 19, no. 10: 3154. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19103154