5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers
Abstract
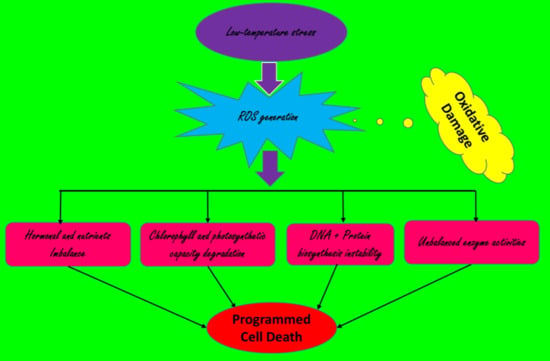
:1. Introduction
2. Results
2.1. The Effect of ALA on Cucumber Seedling Growth and Strong Seedling Index
2.2. The Effect of ALA on Chlorophyll and Photosynthesis
2.3. Effect of ALA on Antioxidant Enzyme Activities, MDA, and ROS Contents
2.4. Effect of ALA on Total Nutrient Contents
2.5. Effect of ALA on Endogenous Hormones Accumulation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Condition
4.2. Treatments and Sampling
4.3. Measurement of Growth Parameters
4.4. Measurement of Chlorophyll Contents
4.5. Measurement of Gas Exchange Parameters
4.6. Leaf Antioxidants Enzymes Activity and MDA Contents
4.7. Determination of H2O2 and O2•− Contents
4.8. Total Nutrients Contents Determination
4.9. Leaf Hormones Extraction and Quantification
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Wu, W.; Zhang, Q.; Ervin, E.H.; Yang, Z.; Zhang, X. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-Epibrassinolide. Front. Plant Sci. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Tang, Y.; Yuan, Y.; Sun, J.; Zhong, M.; Guo, S. The role of 24-epibrassinolide in the regulation of photosynthetic characteristics and nitrogen metabolism of tomato seedlings under a combined low temperature and weak light stress. Plant Physiol. Biochem. 2016, 107, 344. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Emery, R.J.N.; Rahman, M.H.; Kav, N.N.V. A crucial role for cytokinins in pea abr17-mediated enhanced germination and early seedling growth of Arabidopsis under saline and low-temperature stresses. J. Plant Growth Regul. 2007, 26, 26–37. [Google Scholar] [CrossRef]
- Scott, I.M.; Clarke, S.M.; Wood, J.E.; Mur, L.A. Salicylate accumulation inhibits growth at chilling temperature in Arabidopsis. Plant Physiol. 2004, 135, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.P.; Yu, J.Q.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Benefits of brassinosteroid crosstalk. Trends Plant. Sci. 2012, 17, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Vanacker, H.; Gomez, L.D.; Harbinson, J. Regulation of photosynthesis and antioxidant metabolism in maize leaves at optimal and chilling temperatures: review. Plant Physiol. Biochem. 2002, 40, 659–668. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.S.; Jin, Z.L.; Wan, G.L.; Liu, D.; Liu, H.B.; Yoneyama, K.; Zhou, W.J. 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil 2010, 332, 405–415. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Chalkoo, S.; Hayat, S.; Ahmad, A. 28-homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica 2011, 49, 55–64. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Zhao, Z.; An, L. Abscisic acid is involved in brassinosteroids-induced chilling tolerance in the suspension cultured cells from Chorispora bungeana. J. Plant Physiol. 2011, 168, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Yuan, T.; Mao, W.H.; Kai, S.; Asami, T.; Chen, Z.X.; Yu, J.Q. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.L.; Wang, H.C.; Tan, X.Y.; Zhang, C.L.; Naeem, M.S. 5-aminolevulinic acid improves salt tolerance mediated by regulation of tetrapyrrole and proline metabolism in Brassica napus L. seedlings under NaCl stress. Plant Physiol. Biochem. 2018, 124, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, W.; Huang, B. Promotion of photosynthesis by 5-aminolevulinic acid during and after chilling stress in melon seedlings grown under low light condition. Acta Hortic. Sin. 2004, 31, 321–326. [Google Scholar]
- Wu, Y.; Jin, X.; Liao, W.; Hu, L.; Dawuda, M.M.; Zhao, X.; Tang, Z.; Gong, T.; Yu, J. 5-Aminolevulinic Acid (ALA) alleviated salinity stress in cucumber seedlings by enhancing chlorophyll synthesis pathway. Front. Plant Sci. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, L.; Naeem, M.S.; Liu, H.; Deng, X.; Xu, L.; Zhang, F.; Zhou, W. 5-Aminolevulinic acid enhances photosynthetic gas exchange, chlorophyll fluorescence and antioxidant system in oilseed rape under drought stress. Acta Physiol. Plant. 2013, 35, 2747–2759. [Google Scholar] [CrossRef]
- Nguyen, H.; Kim, H.-S.; Jung, S. Altered tetrapyrrole metabolism and transcriptome during growth-promoting actions in rice plants treated with 5-aminolevulinic acid. Plant Growth Regul. 2016, 78, 133–144. [Google Scholar] [CrossRef]
- Naeem, M.S.; Warusawitharana, H.; Liu, H.; Liu, D.; Ahmad, R.; Waraich, E.A.; Xu, L.; Zhou, W. Aminolevulinic acid alleviates the salinity-induced changes in Brassica napus as revealed by the ultrastructural study of chloroplast. Plant Physiol. Biochem. 2012, 57, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.P.; Miao, M.M.; Wang, C.L. Effects of ALA on photosynthesis, antioxidant enzyme activity, and gene expression, and regulation of proline accumulation in tomato seedlings under NaCl stress. J. Plant Growth Regul. 2015, 34, 637–650. [Google Scholar] [CrossRef]
- Zhen, A.; Bie, Z.L.; Huang, Y.; Liu, Z.X.; Fan, M.L. Effects of 5-aminolevulinic acid on the H2O2 content and antioxidative enzyme gene expression in NaCl-treated cucumber seedlings. Biol. Plant. 2012, 56, 566–570. [Google Scholar] [CrossRef]
- Bai, L.; Deng, H.; Zhang, X.; Yu, X.; Li, Y. Gibberellin is involved in inhibition of cucumber growth and nitrogen uptake at suboptimal root-zone temperatures. PLoS ONE 2016, 11, e0156188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Jiang, W.B.; Huang, B.J. Promotion of 5-aminolevulinic acid on photosynthesis of melon (Cucumis melo) seedlings under low light and chilling stress conditions. Physiol. Plant. 2010, 121, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Sun, Y.P.; Zhang, Z.P.; Kang, L.; He, H.H.; Liu, L.W. Effects of 5-aminolevulinic acid (ALA) on photosynthesis and chlorophyll fluorescence of watermelon seedlings grown under low light and low temperature conditions. Acta Hortic. 2010, 856, 159–166. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Yan, F.; Hu, L.P.; Zhou, X.T.; Zou, Z.R.; Cui, L.R. Effects of exogenous 5-aminolevulinic acid on photosynthesis, stomatal conductance, transpiration rate, and PIP gene expression of tomato seedlings subject to salinity stress. Genet. Mol. Res. 2015, 14, 6401–6412. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Wang, B.; Ali, S.; Ghani, M.A.; Hayat, M.T.; Yang, C.; Xu, L.; Zhou, W.J. 5-Aminolevulinic Acid ameliorates the growth, photosynthetic gas exchange capacity, and ultrastructural changes under cadmium stress in Brassica napus L. J. Plant Growth Regul. 2013, 32, 604–614. [Google Scholar] [CrossRef]
- Nishihara, E.; Takahashi, K.; Nakata, N.; Tanaka, K.; Watanabe, K. Effect of 5-Aminolevulinic acid on photosynthetic rate, hydrogen peroxide content, antioxidant level and active oxygen-scavenging enzymes in spinach (Spinacia oleracea L.). J. Jpn. Soc. Hortic. Sci. 2001, 70, 346–352. [Google Scholar] [CrossRef]
- Xia, X.-J.; Huang, L.-F.; Zhou, Y.-H.; Mao, W.-H.; Shi, K.; Wu, J.-X.; Asami, T.; Chen, Z.; Yu, J.-Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in cucumber. Planta 2009, 230, 1185. [Google Scholar] [CrossRef] [PubMed]
- Memon, S.A.; Hou, X.; Wang, L.; Li, Y. Promotive effect of 5-aminolevulinic acid on chlorophyll, antioxidative enzymes and photosynthesis of Pakchoi (Brassica campestris ssp. chinensis var. communis Tsen et Lee). Acta Physiol. Plant. 2009, 31, 51–57. [Google Scholar] [CrossRef]
- Ali, B.; Huang, C.R.; Qi, Z.Y.; Ali, S.; Daud, M.K.; Geng, X.X.; Liu, H.B.; Zhou, W.J. 5-Aminolevulinic acid ameliorates cadmium-induced morphological, biochemical, and ultrastructural changes in seedlings of oilseed rape. Environ. Sci. Pollut. Res. 2013, 20, 7256–7267. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-M.; Zhang, J.; Sun, W.-J.; Li, Q.; Dai, A.-H.; Bai, J.-G. 5-Aminolevulinic acid pretreatment mitigates drought stress of cucumber leaves through altering antioxidant enzyme activity. Sci. Hortic. 2011, 130, 820–828. [Google Scholar] [CrossRef]
- Niu, K.; Ma, H. The positive effects of exogenous 5-aminolevulinic acid on the chlorophyll biosynthesis, photosystem and calvin cycle of Kentucky bluegrass seedlings in response to osmotic stress. Environ. Exp.Bot. 2018, 155, 260–271. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, S.; Wang, J.; Su, X.; Suo, B.; Qin, F.; Zhao, H. Exogenous application of 5-aminolevulinic acid on wheat seedlings under drought stress enhances the transcription of psbA and psbD genes and improves photosynthesis. Braz. J. Bot. 2018, 41, 275–285. [Google Scholar] [CrossRef]
- Alikhani-Koupaei, M.; Fatahi, R.; Zamani, Z.; Salimi, S. 5-Aminolevulinic acid moderates environmental stress-induced bunch wilting and stress markers in date palm. Acta Physiol. Plant. 2018, 40, 159. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation under Abiotic Stress; Springer: Singapore, 2017. [Google Scholar]
- Kanto, U.; Jutamanee, K.; Osotsapar, Y.; Chai-arree, W.; Jattupornpong, S. Promotive Effect of Priming with 5-Aminolevulinic Acid on Seed Germination Capacity, Seedling Growth and Antioxidant EnzymeActivity in Rice Subjected to Accelerated Ageing Treatment. Plant Prod. Sci. 2015, 18, 443–454. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tanaka, A.; Tsuji, H. Effects of 5-aminolevulinic acid on the accumulation of chlorophyll b and apoproteins of the light-harvesting chlorophyll a/b-protein complex of photosystem II. Plant Cell Physiol. 1993, 34, 465–472. [Google Scholar]
- Fariduddin, Q.; Khalil, R.R.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 2013, 185, 7845–7856. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Korkmaz, Y.; Demirkıran, A.R. Enhancing chilling stress tolerance of pepper seedlings by exogenous application of 5-aminolevulinic acid. Environ. Exp. Bot. 2010, 67, 495–501. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Gu, W.; Zhang, Q.; Tian, L.; Guo, S.; Wei, S. Exogenous application of 5-aminolevulinic acid improves low-temperature stress tolerance of maize seedlings. Crop Pasture Sci. 2018, 69, 587–593. [Google Scholar] [CrossRef]
- Ali, B.; Xu, X.; Gill, R.A.; Yang, S.; Ali, S.; Tahir, M.; Zhou, W. Promotive role of 5-aminolevulinic acid on mineral nutrients and antioxidative defense system under lead toxicity in Brassica napus. Ind. Crop Prod. 2014, 52, 617–626. [Google Scholar] [CrossRef]
- Balestrasse, K.B.; Tomaro, M.L.; Batlle, A.; Noriega, G.O. The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 2010, 71, 2038–2045. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, L.Y.; Ali, B.; Yang, A.G.; Wan, G.L.; Xu, L.; Zhou, W.J. Influence of 5-aminolevulinic acid on photosynthetically related parameters and gene expression in Brassica napus L. under drought stress. Soil Sci. Plant Nutr. 2016, 62, 254–262. [Google Scholar] [CrossRef]
- Anjum, S.A.; Niu, J.H.; Wang, R.; Li, J.H.; Liu, M.R.; Song, J.X.; Lu, J.; Zohaib, A.; Wang, S.G.; Zong, X.F. Regulation Mechanism of Exogenous 5-Aminolevulinic Acid on Growth and Physiological Characters of Leymus chinensis (Trin.) under High Temperature Stress. Philipp. Agric. Sci. 2016, 99, 253–259. [Google Scholar]
- Liu, D.; Kong, D.D.; Fu, X.K.; Ali, B.; Xu, L.; Zhou, W.J. Influence of exogenous 5-aminolevulinic acid on chlorophyll synthesis and related gene expression in oilseed rape de-etiolated cotyledons under water-deficit stress. Photosynthetica 2016, 54, 468–474. [Google Scholar] [CrossRef]
- Sheteiwy, M.; Shen, H.; Xu, J.; Guan, Y.; Song, W.; Hu, J. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017, 137, 58–72. [Google Scholar] [CrossRef]
- Kočová, M.; Rothová, O.; Holá, D.; Kvasnica, M.; Kohout, L. The effects of brassinosteroids on photosynthetic parameters in leaves of two field-grown maize inbred lines and their F1 hybrid. Biol. Plant. 2010, 54, 785–788. [Google Scholar] [CrossRef]
- Wenwen, W.U.; Yuyan, A.N.; Wang, L. Study on Time Effects of Exogenous 5-Aminolevulinic Acid Treatment on Alleviating Salinity Injury in ‘Benihoppe’ Strawberry. Acta Hortic. Sin. 2017, 44, 1038–1048. [Google Scholar]
- Phung, T.-H.; Jung, S. Differential antioxidant defense and detoxification mechanisms in photodynamically stressed rice plants treated with the deregulators of porphyrin biosynthesis, 5-aminolevulinic acid and oxyfluorfen. Biochem. Biophys. Res. Commun. 2015, 459, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Leigh, R. Ion Homeostasis. In Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation; Pareek, A., Sopory, S.K., Bohnert, H.J., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 245–262. [Google Scholar]
- Yin, D.; Zhang, J.; Jing, R.; Qu, Q.; Guan, H.; Zhang, L.; Dong, L. Effect of salinity on ion homeostasis in three halophyte species, Limonium bicolor, Vitex trifolia Linn. var. simplicifolia Cham and Apocynaceae venetum. Acta Physiol. Plant. 2018, 40, 40. [Google Scholar] [CrossRef]
- Zhu, J.-K. Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 2003, 6, 441–445. [Google Scholar] [CrossRef]
- Jiang, C.; Cui, Q.; Feng, K.; Xu, D.; Li, C.; Zheng, Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 82. [Google Scholar] [CrossRef]
- Chen, G.; Fan, P.S.; Feng, W.M.; Guan, A.Q.; Lu, Y.Y.; Wan, Y.L. Effects of 5-aminolevulinic acid on nitrogen metabolism and ion distribution of watermelon seedlings under salt stress. Russ. J. Plant Physiol. 2017, 64, 116–123. [Google Scholar] [CrossRef]
- Tang, X.-Q.; Wang, Y.; Lv, T.-T.; Xiao, Y.-H. Role of 5-aminolevulinic acid on growth, photosynthetic parameters and antioxidant enzyme activity in NaCl-stressed Isatis indigotica Fort. Russ. J. Plant Physol. 2017, 64, 198–206. [Google Scholar] [CrossRef]
- Marzec, M.; Alqudah, A.M. Key Hormonal components regulate agronomically important traits in Barley. Int. J. Mol. Sci. 2018, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide Ameliorates Endogenous Hormone Levels to Enhance Low-Temperature Stress Tolerance in Cucumber Seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Deng, X.G.; Zhu, T.; Zheng, T.; Li, P.X.; Wu, J.Q.; Zhang, D.W.; Lin, H.H. Ethylene is Involved in Brassinosteroids Induced Alternative Respiratory Pathway in Cucumber (Cucumis sativus L.) Seedlings Response to Abiotic Stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef] [PubMed]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant Hormone Homeostasis, Signaling, and Function during Adventitious Root Formation in Cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Nasir, T.; Abbasi, G.H.; Mia, R.; Ata-Ul-Karim, S.T.; Sah, B. Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol. Environ. Saf. 2018, 151, 255. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pei, Z.F.; Naeem, M.S.; Ming, D.F.; Liu, H.B.; Khan, F.; Zhou, W.J. 5-Aminolevulinic Acid Activates Antioxidative Defence System and Seedling Growth in Brassica napus L. under Water-Deficit Stress. J. Agron. Crop Sci. 2011, 197, 284–295. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Mu, Y.; Anwar, A.; He, C.; Yan, Y.; Li, Y.; Yu, X. Heterotrimeric G-Protein γ Subunit CsGG3.2 Positively Regulates the Expression of CBF Genes and Chilling Tolerance in Cucumber. Front. Plant Sci. 2018, 9, 488. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Yi, G.X.; Wang, B.M.; Deng, A.X.; Nan, T.G.; Li, Z.H.; Li, Q.X. Comparison between conventional indirect competitive enzyme-linked immunosorbent assay (icELISA) and simplified icELISA for small molecules. Anal. Chim. Acta 2006, 571, 79. [Google Scholar] [CrossRef] [PubMed]







| No. | Hypocotyl (mm) | Root Length (cm) | Height (cm) | Leaf Area (cm2) | Total DW (g) | SSI |
|---|---|---|---|---|---|---|
| RT | 5.01 ± 0.12 a | 17.50 ± 1.15 a | 7.50 ± 0.32 a | 97.21 ± 6.31 a | 0.74 ± 0.10 a | 0.57 ± 0.04 a |
| CK | 3.68 ± 0.06 c | 10.13 ± 0.85 c | 4.37 ± 0.25 c | 53.68 ± 2.83 c | 0.38 ± 0.03 d | 0.36 ± 0.03 c |
| T1 | 3.76 ± 0.12 c | 10.88 ± 0.85 c | 4.63 ± 0.47 c | 50.42 ± 5.01 c | 0.44 ± 0.03 c | 0.41 ± 0.04 c |
| T2 | 4.75 ± 0.23 b | 14.15 ± 1.07 b | 6.38 ± 0.25 b | 78.81 ± 4.51 b | 0.56 ± 0.03 b | 0.51 ± 0.05 b |
| T3 | 4.72 ± 0.29 b | 14.25 ± 0.95 b | 6.13 ± 0.25 b | 73.56 ± 4.84 b | 0.53 ± 0.04 b | 0.49 ± 0.02 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113379
Anwar A, Yan Y, Liu Y, Li Y, Yu X. 5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers. International Journal of Molecular Sciences. 2018; 19(11):3379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113379
Chicago/Turabian StyleAnwar, Ali, Yan Yan, Yumei Liu, Yansu Li, and Xianchang Yu. 2018. "5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers" International Journal of Molecular Sciences 19, no. 11: 3379. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19113379