From mRNA Expression of Drug Disposition Genes to In Vivo Assessment of CYP-Mediated Biotransformation during Zebrafish Embryonic and Larval Development
Abstract
:1. Introduction
2. Results
2.1. In Vitro Study on Cytochrome P450 Activity in Zebrafish Embryos, Larvae and Adults
2.2. In Vivo Study on Cytochrome P450 Activity in Zebrafish Embryos and Larvae
2.2.1. Quantitative Analysis of Resorufin Formation
2.2.2. Qualtitative Analysis of Resorufin Formation
2.3. mRNA Expression of Phase I and Phase II Enzymes and P–Glycoprotein
3. Discussion
3.1. Ontogeny of In Vitro and In Vivo Cytochrome P450 Activity in Zebrafish Embryos, Larvae and Adults
3.1.1. In Vitro versus In Vivo
3.1.2. Benzyloxy-Methyl-Resorufin versus 7–Ethoxyresorufin
3.1.3. Literature versus Current Study
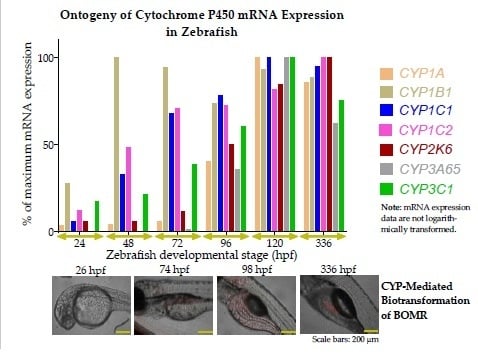
3.2. Ontogeny of Cytochrome P450 mRNA Expression in Zebrafish Embryos and Larvae
3.2.1. Cytochrome P450 mRNA Expression during Zebrafish Organogenesis
3.2.2. Cytochrome P450 mRNA Expression during Zebrafish Larval Development
3.3. Ontogeny of mRNA Expression of Two Phase II enzymes and a P–glycoprotein in Zebrafish Embryos and Larvae
4. Materials and Methods
4.1. In Vitro Study on Cytochrome P450 Activity in Zebrafish Embryos, Larvae and Adults
4.1.1. Fish Maintenance and Breeding
- Fish Maintenance and Breeding: Zebrafish Embryos
- Fish Maintenance and Breeding: Zebrafish Larvae
- Fish Maintenance: Adult Zebrafish
4.1.2. Tissue Collection and Isolation of Microsomes
- Isolation of Microsomes from Whole Zebrafish Embryos
- Isolation of Microsomes from Whole Zebrafish Larvae
- Isolation of Microsomes from Whole Adult Zebrafish
4.1.3. Benzyloxy-Methyl-Resorufin Assay in Microsomes Prepared from Whole Zebrafish Embryos, Larvae and Adults
4.1.4. Mathematical and Statistical Analyses
4.2. In Vivo Study on Cytochrome P450 Activity in Zebrafish Embryos and Larvae
4.2.1. Fish Maintenance and Breeding
4.2.2. Benzyloxy-Methyl-Resorufin Assay in Zebrafish Embryos and Larvae
4.2.3. Mathematical and Statistical Analyses
4.3. mRNA Expression of Phase I and Phase II Enzymes and P–Glycoprotein
4.3.1. Fish Maintenance and Breeding
4.3.2. Quantification of mRNA Levels by means of qPCR
4.3.3. Mathematical and Statistical Analyses.
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | ATP-binding cassette |
| AhR | Aryl hydrocarbon receptor |
| BOMR | Benzyloxy-methyl-resorufin |
| CRO | Contract research organization |
| CYP | Cytochrome P450 |
| Dpf | Days post-fertilization |
| ER | 7-Ethoxyresorufin |
| EROD | Ethoxyresorufin-o-deethylase |
| FET | Fish embryo acute toxicity test |
| FMO | Flavin monooxygenase |
| Hpf | Hours post-fertilization |
| LLOD | Lower limit of detection |
| LLOQ | Lower limit of quantification |
| MP | Microsomal protein |
| MXR | Multixenobiotic resistance |
| OECD | Organization for Economic Co-operation and Development |
| PXR | Pregnane X receptor |
| qPCR | Quantitative polymerase chain reaction |
| RA | Retinoic acid |
| S.B. | Swim bladder |
| SULT | Sulfotransferase |
| TCDD | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
| UGT | Uridine diphosphate glucuronosyltransferase |
| ZEDTA | Zebrafish embryo developmental toxicity assay |
| ZEM | Microsomes prepared from whole zebrafish embryo homogenates |
| ZLaM | Microsomes prepared from whole zebrafish larva homogenates |
| ZLM | Zebrafish liver microsomes |
| ZM | Microsomes prepared from whole adult zebrafish homogenates |
References
- Knudsen, T.B.; Martin, M.T.; Kavlock, R.J.; Judson, R.S.; Dix, D.J.; Singh, A.V. Profiling the activity of environmental chemicals in prenatal developmental toxicity studies using the U.S. EPA’s ToxRefDB. Reprod. Toxicol. 2009, 28, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, P.T.; Beken, S.; Beyer, B.K.; Breslin, W.J.; Cappon, G.D.; Chen, C.L.; Chmielewski, G.; De Schaepdrijver, L.; Enright, B.; Foreman, J.E.; et al. Comparison of rat and rabbit embryo-fetal developmental toxicity data for 379 pharmaceuticals: On the nature and severity of developmental effects. Crit. Rev. Toxicol. 2016, 46, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; ISBN 9780900767784. [Google Scholar]
- European Union. 2012/707/EU: Commission Implementing Decision of 14 November 2012 establishing a common format for the submission of the information pursuant to Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes (notified under document c(2012) 8064). Off. J. Eur. Union 2012, 320, 33–50. [Google Scholar]
- Strahle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish embryos as an alternative to animal experiments—A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Alderton, W.K. Zebrafish in pharmaceutical industry research: Finding the best fit. Drug Discov. Today Dis. Models 2013, 10, e43–e50. [Google Scholar] [CrossRef]
- Ball, J.S.; Stedman, D.B.; Hillegass, J.M.; Zhang, C.X.; Panzica-Kelly, J.; Coburn, A.; Enright, B.P.; Tornesi, B.; Amouzadeh, H.R.; Hetheridge, M.; et al. Fishing for teratogens: A consortium effort for a harmonized zebrafish developmental toxicology assay. Toxicol. Sci. 2014, 139, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, A.L.; Stedman, D.B.; Ball, J.; Hillegass, J.M.; Flood, A.; Zhang, C.X.; Panzica-Kelly, J.; Cao, J.; Coburn, A.; Enright, B.P.; et al. Inter-laboratory assessment of a harmonized zebrafish developmental toxicology assay—Progress report on phase I. Reprod. Toxicol. 2012, 33, 155–164. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test. In OECD Guidelines for the Testing of Chemicals; 2013; ISBN 9789264203709. Available online: https://0-doi-org.brum.beds.ac.uk/10.1787/9789264203709-en (accessed on 6 August 2018).
- OECD. Test No. 203: Fish, Acute Toxicity Test. In OECD Guideline for the Testing of Chemicals; 1992; ISBN 978926406996. Available online: https://0-doi-org.brum.beds.ac.uk/10.1787/9789264069961-en (accessed on 6 August 2018).
- Sobanska, M.; Scholz, S.; Nyman, A.M.; Cesnaitis, R.; Gutierrez Alonso, S.; Kluver, N.; Kuhne, R.; Tyle, H.; de Knecht, J.; Dang, Z.; et al. Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of Registration, Evaluation, Authorisation, and Restriction of chemicals (REACH). Environ. Toxicol. Chem. SETAC 2018, 37, 657–670. [Google Scholar] [CrossRef]
- Beekhuijzen, M.; de Koning, C.; Flores-Guillen, M.E.; de Vries-Buitenweg, S.; Tobor-Kaplon, M.; van de Waart, B.; Emmen, H. From cutting edge to guideline: A first step in harmonization of the zebrafish embryotoxicity test (ZET) by describing the most optimal test conditions and morphology scoring system. Reprod. Toxicol. 2015, 56, 64–76. [Google Scholar] [CrossRef]
- Brannen, K.C.; Panzica-Kelly, J.M.; Danberry, T.L.; Augustine-Rauch, K.A. Development of a zebrafish embryo teratogenicity assay and quantitative prediction model. Birth Defects Res. B Dev. Reprod. Toxicol. 2010, 89, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Field, H.A.; Ober, E.A.; Roeser, T.; Stainier, D.Y. Formation of the digestive system in zebrafish. I. Liver morphogenesis. Dev. Biol. 2003, 253, 279–290. [Google Scholar] [CrossRef]
- Ng, A.N.; de Jong-Curtain, T.A.; Mawdsley, D.J.; White, S.J.; Shin, J.; Appel, B.; Dong, P.D.; Stainier, D.Y.; Heath, J.K. Formation of the digestive system in zebrafish: III. Intestinal epithelium morphogenesis. Dev. Biol. 2005, 286, 114–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ober, E.A.; Field, H.A.; Stainier, D.Y. From endoderm formation to liver and pancreas development in zebrafish. Mech. Dev. 2003, 120, 5–18. [Google Scholar] [CrossRef]
- Kais, B.; Schneider, K.E.; Keiter, S.; Henn, K.; Ackermann, C.; Braunbeck, T. DMSO modifies the permeability of the zebrafish (Danio rerio) chorion-implications for the fish embryo test (FET). Aquat. Toxicol. 2013, 140, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.W.; Kam, P.C. The physiological and pharmacological roles of cytochrome P450 isoenzymes. Anaesthesia 1999, 54, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guengerich, F.P. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006, 8, E101–E111. [Google Scholar] [CrossRef] [PubMed]
- Martignoni, M.; Groothuis, G.M.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jonsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef]
- Hakkola, J.; Pasanen, M.; Purkunen, R.; Saarikoski, S.; Pelkonen, O.; Maenpaa, J.; Rane, A.; Raunio, H. Expression of xenobiotic-metabolizing cytochrome P450 forms in human adult and fetal liver. Biochem. Pharmacol. 1994, 48, 59–64. [Google Scholar] [CrossRef]
- Rich, K.J.; Boobis, A.R. Expression and inducibility of P450 enzymes during liver ontogeny. Microsc. Res. Tech. 1997, 39, 424–435. [Google Scholar] [CrossRef]
- Verbueken, E.; Alsop, D.; Saad, M.A.; Pype, C.; Van Peer, E.M.; Casteleyn, C.R.; Van Ginneken, C.J.; Wilson, J.; Van Cruchten, S.J. In vitro biotransformation of two human CYP3A probe substrates and their inhibition during early zebrafish development. Int. J. Mol. Sci. 2017, 18, 217. [Google Scholar] [CrossRef]
- Braunig, J.; Schiwy, S.; Broedel, O.; Muller, Y.; Frohme, M.; Hollert, H.; Keiter, S.H. Time-dependent expression and activity of cytochrome P450 1s in early life-stages of the zebrafish (Danio rerio). Environ. Sci. Pollut. Res. Int. 2015, 22, 16319–16328. [Google Scholar] [CrossRef] [PubMed]
- Chng, H.T.; Ho, H.K.; Yap, C.W.; Lam, S.H.; Chan, E.C. An investigation of the bioactivation potential and metabolism profile of zebrafish versus human. J. Biomol. Screen. 2012, 17, 974–986. [Google Scholar] [CrossRef] [PubMed]
- Creusot, N.; Brion, F.; Piccini, B.; Budzinski, H.; Porcher, J.M.; Ait-Aissa, S. BFCOD activity in fish cell lines and zebrafish embryos and its modulation by chemical ligands of human aryl hydrocarbon and nuclear receptors. Environ. Sci. Pollut. Res. Int. 2015, 22, 16393–16404. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.S.; Panter, G.H.; Hutchinson, T.H.; Chipman, J.K. Oxidative and conjugative xenobiotic metabolism in zebrafish larvae in vivo. Zebrafish 2010, 7, 23–30. [Google Scholar] [CrossRef]
- Kais, B.; Schiwy, S.; Hollert, H.; Keiter, S.H.; Braunbeck, T. In vivo EROD assays with the zebrafish (Danio rerio) as rapid screening tools for the detection of dioxin-like activity. Sci. Total Environ. 2017, 590, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Nie, F.H.; Lin, H.Y.; Ma, Y.; Ju, X.H.; Chen, J.J.; Gooneratne, R. Developmental toxicity, EROD, and CYP1A mRNA expression in zebrafish embryos exposed to dioxin-like PCB126. Environ. Toxicol. 2016, 31, 201–210. [Google Scholar] [CrossRef]
- Mattingly, C.J.; Toscano, W.A. Posttranscriptional silencing of cytochrome P4501A1 (CYP1A1) during zebrafish (Danio rerio) development. Dev. Dyn. 2001, 222, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Noury, P.; Geffard, O.; Tutundjian, R.; Garric, J. Non destructive in vivo measurement of ethoxyresorufin biotransformation by zebrafish prolarva: Development and application. Environ. Toxicol. 2006, 21, 324–331. [Google Scholar] [CrossRef]
- Otte, J.C.; Schmidt, A.D.; Hollert, H.; Branbeck, T. Spatio-temporal development of CYP1 activity in early life-stages of zebrafish (Danio rerio). Aquat. Toxicol. 2010, 100, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Otte, J.C.; Schultz, B.; Fruth, D.; Fabian, E.; van Ravenzwaay, B.; Hidding, B.; Salinas, E.R. Intrinsic xenobiotic metabolizing enzyme activities in early life stages of zebrafish (Sanio rerio). Toxicol. Sci. 2017, 159, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Oziolor, E.M.; Carey, A.N.; Matson, C.W. A non-destructive BFCOD assay for in vivo measurement of cytochrome P450 3A (CYP3A) enzyme activity in fish embryos and larvae. Ecotoxicology 2017, 26, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Verbueken, E.; Pype, C.; Casteleyn, C.; Van Ginneken, C.; Maes, L.; Cos, P.; Van Cruchten, S. In vitro CYP1A activity in the zebrafish: Temporal but low metabolite levels during organogenesis and lack of gender differences in the adult stage. Reprod. Toxicol. 2016, 64, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Schiwy, S.; Braunig, J.; Alert, H.; Hollert, H.; Keiter, S.H. A novel contact assay for testing aryl hydrocarbon receptor (AhR)-mediated toxicity of chemicals and whole sediments in zebrafish (Danio rerio) embryos. Environ. Sci. Pollut. Res. Int. 2015, 22, 16305–16318. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.; Berghmans, S.; Butler, P.; Chassaing, H.; Fleming, A.; Golder, Z.; Richards, F.; Gardner, I. Accumulation and metabolism of drugs and CYP probe substrates in zebrafish larvae. Xenobiotica 2010, 40, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Zebrafish (Danio rerio) embryos as a model for testing proteratogens. Toxicology 2011, 281, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Karchner, S.I.; Franks, D.G.; Hahn, M.E. AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: Tandem arrangement of ahr1b and ahr2 genes. Biochem. J. 2005, 392, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Goldstone, J.V.; Lemaire, B.; Takata, M.; Woodin, B.R.; Stegeman, J.J. Role of pregnane X receptor and aryl hydrocarbon receptor in transcriptional regulation of pxr, CYP2, and CYP3 genes in developing zebrafish. Toxicol. Sci. 2015, 143, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Aspects of larval rearing. ILAR J. 2012, 53, 169–178. [Google Scholar] [CrossRef]
- Billiard, S.M.; Timme-Laragy, A.R.; Wassenberg, D.M.; Cockman, C.; Di Giulio, R.T. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006, 92, 526–536. [Google Scholar] [CrossRef]
- Nacci, D.; Coiro, L.; Kuhn, A.; Champlin, D.; Munns, W.; Specker, J.; Cooper, K. Nondestructive indicator of ethoxyresorufin-o-deethylase activity in embryonic fish. Environ. Toxicol. Chem. 1998, 17, 2481–2486. [Google Scholar] [CrossRef]
- Chang, T.K.; Waxman, D.J. Enzymatic analysis of cDNA-expressed human CYP1A1, CYP1A2, and CYP1B1 with 7-ethoxyresorufin as substrate. Methods Mol. Biol. 2006, 320, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Luckenbach, T.; Fischer, S.; Sturm, A. Current advances on ABC drug transporters in fish. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 165, 28–52. [Google Scholar] [CrossRef] [PubMed]
- Christen, V.; Fent, K. Tissue-, sex- and development-specific transcription profiles of eight UDP-glucuronosyltransferase genes in zebrafish (Danio rerio) and their regulation by activator of aryl hydrocarbon receptor. Aquat. Toxicol. 2014, 150, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.A.; Faletto, M.B. Advances in mechanisms of activation and deactivation of environmental chemicals. Environ. Health Perspect. 1993, 100, 169–176. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Q. Cloning and comparative analyses of the zebrafish Ugt repertoire reveal its evolutionary diversity. PLoS ONE 2010, 5, e9144. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yang, Y.S.; Sugahara, T.; Yasuda, S.; Liu, M.C. Identification of a novel zebrafish SULT1 cytosolic sulfotransferase: Cloning, expression, characterization, and developmental expression study. Arch. Biochem. Biophys. 2005, 437, 10–19. [Google Scholar] [CrossRef]
- Riordan, J.R.; Deuchars, K.; Kartner, N.; Alon, N.; Trent, J.; Ling, V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature 1985, 316, 817–819. [Google Scholar] [CrossRef]
- Fischer, S.; Kluver, N.; Burkhardt-Medicke, K.; Pietsch, M.; Schmidt, A.M.; Wellner, P.; Schirmer, K.; Luckenbach, T. Abcb4 acts as multixenobiotic transporter and active barrier against chemical uptake in zebrafish (Danio rerio) embryos. BMC Biol. 2013, 11, 69. [Google Scholar] [CrossRef]
- Vergauwen, L.; Cavallin, J.E.; Ankley, G.T.; Bars, C.; Gabriels, I.J.; Michiels, E.D.G.; Fitzpatrick, K.R.; Periz-Stanacev, J.; Randolph, E.C.; Robinson, S.L.; et al. Gene transcription ontogeny of hypothalamic-pituitary-thyroid axis development in early-life stage fathead minnow and zebrafish. Gen. Comp. Endocrinol. 2018, 266, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, E.A.; Spitsbergen, J.M.; Tanguay, R.L.; Stegeman, J.J.; Heideman, W.; Peterson, R.E. Tissue-specific expression of AHR2, ARNT2, and CYP1A in zebrafish embryos and larvae: Effects of developmental stage and 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure. Toxicol. Sci. 2002, 68, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Corley-Smith, G.E.; Su, H.T.; Wang-Buhler, J.L.; Tseng, H.P.; Hu, C.H.; Hoang, T.; Chung, W.G.; Buhler, D.R. CYP3C1, the first member of a new cytochrome P450 subfamily found in zebrafish (Danio rerio). Biochem. Biophys. Res. Commun. 2006, 340, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, K.; Wang, S.; Hou, H.; Yuan, Y.; Wang, X. Toxicity induced by emodin on zebrafish embryos. Drug Chem. Toxicol. 2012, 35, 149–154. [Google Scholar] [CrossRef]
- Jackson, J.S.; Kennedy, C.J. Regulation of hepatic abcb4 and cyp3a65 gene expression and multidrug/multixenobiotic resistance (MDR/MXR) functional activity in the model teleost, Danio rerio (zebrafish). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 200, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, A.; Vogs, C.; Seiwert, B.; Aulhorn, S.; Altenburger, R.; Hollert, H.; Kuster, E.; Busch, W. Biotransformation in the zebrafish embryo -temporal gene transcription changes of cytochrome P450 enzymes and internal exposure dynamics of the AhR binding xenobiotic benz[a]anthracene. Environ. Pollut. 2017, 230, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, J.; Fang, Q.; Li, Y. Transcription alterations of microRNAs, cytochrome P4501A1 and 3A65, and AhR and PXR in the liver of zebrafish exposed to crude microcystins. Toxicon 2013, 73, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Shaya, L.; Dejong, C.; Wilson, J.Y. Expression patterns of cytochrome P450 3B and 3C genes in model fish species. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 166, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.P.; Hseu, T.H.; Buhler, D.R.; Wang, W.D.; Hu, C.H. Constitutive and xenobiotics-induced expression of a novel CYP3A gene from zebrafish larva. Toxicol. Appl. Pharmacol. 2005, 205, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wang-Buhler, J.L.; Lee, S.J.; Chung, W.G.; Stevens, J.F.; Tseng, H.P.; Hseu, T.H.; Hu, C.H.; Westerfield, M.; Yang, Y.H.; Miranda, C.L.; et al. CYP2K6 from zebrafish (Danio rerio): Cloning, mapping, developmental/tissue expression, and aflatoxin B1 activation by baculovirus expressed enzyme. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 207–219. [Google Scholar] [CrossRef]
- Parichy, D.M.; Elizondo, M.R.; Mills, M.G.; Gordon, T.N.; Engeszer, R.E. Normal table of postembryonic zebrafish development: Staging by externally visible anatomy of the living fish. Dev. Dyn. 2009, 238, 2975–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.; Wang, M.; Gui, W.; Zhao, Y.; Yu, L.; Zhu, G. Changes in thyroid hormone levels during zebrafish development. Zool. Sci. 2012, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Drummond, I.A.; Majumdar, A.; Hentschel, H.; Elger, M.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Stemple, D.L.; Zwartkruis, F.; Rangini, Z.; et al. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 1998, 125, 4655–4667. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Joseph, N.M.; Easter, S.S. The morphogenesis of the zebrafish eye, including a fate map of the optic vesicle. Dev. Dyn. 2000, 218, 175–188. [Google Scholar] [CrossRef] [Green Version]
- Wallace, K.N.; Pack, M. Unique and conserved aspects of gut development in zebrafish. Dev. Biol. 2003, 255, 12–29. [Google Scholar] [CrossRef] [Green Version]
- Paine, M.F.; Hart, H.L.; Ludington, S.S.; Haining, R.L.; Rettie, A.E.; Zeldin, D.C. The human intestinal cytochrome P450 “pie”. Drug Metab. Dispos. 2006, 34, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Radominska-Pandya, A.; Czernik, P.J.; Little, J.M.; Battaglia, E.; Mackenzie, P.I. Structural and functional studies of UDP-glucuronosyltransferases. Drug Metab. Rev. 1999, 31, 817–899. [Google Scholar] [CrossRef]
- Rettie, A.E.; Fisher, M.B. Handbook of Drug Metabolism; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- Jonsson, M.E.; Orrego, R.; Woodin, B.R.; Goldstone, J.V.; Stegeman, J.J. Basal and 3,3’,4,4’,5-pentachlorobiphenyl-induced expression of cytochrome P450 1A, 1B and 1C genes in zebrafish. Toxicol. Appl. Pharmacol. 2007, 221, 29–41. [Google Scholar] [CrossRef]
- Chambers, D.; Wilson, L.; Maden, M.; Lumsden, A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development 2007, 134, 1369–1383. [Google Scholar] [CrossRef] [Green Version]
- Romand, R.; Dolle, P.; Hashin, E. Retinoid signaling in inner ear development. J. Neurobiol. 2006, 66, 687–704. [Google Scholar] [CrossRef] [Green Version]
- Thatcher, J.E.; Isoherranen, N. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin. Drug Metab. Toxicol. 2009, 5, 875–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saitou, M.; Sugai, S.; Tanaka, T.; Shimouchi, K.; Fuchs, E.; Narumiya, S.; Kakizuka, A. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature 1995, 374, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Pelegri, F. Maternal factors in zebrafish development. Dev. Dyn. 2003, 228, 535–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, H.C.; Tseng, H.P.; Chung, H.Y.; Ko, C.Y.; Tzou, W.S.; Buhler, D.R.; Hu, C.H. Influence of TCDD on zebrafish CYP1B1 transcription during development. Toxicol. Sci. 2008, 103, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Hakkola, J.; Pasanen, M.; Pelkonen, O.; Hukkanen, J.; Evisalmi, S.; Anttila, S.; Rane, A.; Mantyla, M.; Purkunen, R.; Saarikoski, S.; et al. Expression of CYP1B1 in human adult and fetal tissues and differential inducibility of CYP1B1 and CYP1A1 by Ah receptor ligands in human placenta and cultured cells. Carcinogenesis 1997, 18, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yelon, D.; Horne, S.A.; Stainier, D.Y. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 1999, 214, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Glisic, B.; Hrubik, J.; Fa, S.; Dopudj, N.; Kovacevic, R.; Andric, N. Transcriptional profiles of glutathione-S-transferase isoforms, Cyp, and AOE genes in atrazine-exposed zebrafish embryos. Environ. Toxicol. 2016, 31, 233–244. [Google Scholar] [CrossRef]
- Maack, G.; Segner, H. Morphological development of the gonads in zebrafish. J. Fish Biol. 2003, 62, 895–906. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Requeni, P.; Conceicao, L.E.C.; Jordal, A.E.O.; Ronnestad, I. A reference growth curve for nutritional experiments in zebrafish (Danio rerio) and changes in whole body proteome during development. Fish Physiol. Biochem. 2010, 36, 1199–1215. [Google Scholar] [CrossRef]
- Penner, N. Appendix: Drug metabolizing enzymes and biotransformation reactions. In ADME-Enabling Technologies in Drug Design and Development; Zhang, D., Surapaneni, S., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; ISBN 9780470542781. [Google Scholar]
- Liu, T.A.; Bhuiyan, S.; Liu, M.Y.; Sugahara, T.; Sakakibara, Y.; Suiko, M.; Yasuda, S.; Kakuta, Y.; Kimura, M.; Williams, F.E.; et al. Zebrafish as a model for the study of the phase II cytosolic sulfotransferases. Curr. Drug Metab. 2010, 11, 538–546. [Google Scholar] [CrossRef]
- Valentim, A.M.; van Eeden, F.J.; Strahle, U.; Olsson, I.A. Euthanizing zebrafish legally in Europe: Are the approved methods of euthanizing zebrafish appropriate to research reality and animal welfare? EMBO Rep. 2016, 17, 1688–1689. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio), 5th ed.; M. Westerfield: Eugene, OR, USA, 2007; Available online: https://zfin.org/zf_info/zfbook/zfbk.html (accessed on 6 September 2018).
- Sengul, U. Comparing determination methods of detection and quantification limits for aflatoxin analysis in hazelnut. J. Food Drug Anal. 2016, 24, 56–62. [Google Scholar] [CrossRef] [PubMed]
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Henn, K.; Braunbeck, T. Dechorionation as a tool to improve the fish embryo toxicity test (FET) with the zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 91–98. [Google Scholar] [CrossRef] [PubMed]
- NCBI, BLAST. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 6 September 2018).
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef] [Green Version]
- Olsvik, P.A.; Williams, T.D.; Tung, H.S.; Mirbahai, L.; Sanden, M.; Skjaerven, K.H.; Ellingsen, S. Impacts of TCDD and MeHg on DNA methylation in zebrafish (Danio rerio) across two generations. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 165, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Chung, H.Y.; Su, H.T.; Tseng, H.P.; Tzou, W.S.; Hu, C.H. Regulation of zebrafish CYP3A65 transcription by AHR2. Toxicol. Appl. Pharmacol. 2013, 270, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Fang, X.; Zhang, H.; Dai, J. The thyroid-disrupting effects of long-term perfluorononanoate exposure on zebrafish (Danio rerio). Ecotoxicology 2011, 20, 47–55. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Wu, Q. Characterization of the zebrafish Ugt repertoire reveals a new class of drug-metabolizing UDP glucuronosyltransferases. Mol. Pharmacol. 2014, 86, 62–75. [Google Scholar] [CrossRef]
- Biga, P.R.; Roberts, S.B.; Iliev, D.B.; McCauley, L.A.; Moon, J.S.; Collodi, P.; Goetz, F.W. The isolation, characterization, and expression of a novel GDF11 gene and a second myostatin form in zebrafish, Danio rerio. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 141, 218–230. [Google Scholar] [CrossRef]
- Gonzalez, P.; Baudrimont, M.; Boudou, A.; Bourdineaud, J.P. Comparative effects of direct cadmium contamination on gene expression in gills, liver, skeletal muscles and brain of the zebrafish (Danio rerio). Biometals 2006, 19, 225–235. [Google Scholar] [CrossRef]
- De Wit, M.; Keil, D.; Remmerie, N.; van der Ven, K.; van den Brandhof, E.J.; Knapen, D.; Witters, E.; De Coen, W. Molecular targets of TBBPA in zebrafish analysed through integration of genomic and proteomic approaches. Chemosphere 2008, 74, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Houbrechts, A.M.; Vergauwen, L.; Bagci, E.; Van Houcke, J.; Heijlen, M.; Kulemeka, B.; Hyde, D.R.; Knapen, D.; Darras, V.M. Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function. Mol. Cell. Endocrinol. 2016, 424, 81–93. [Google Scholar] [CrossRef] [PubMed]







| Experimental Setup | In Vitro | In Vivo |
|---|---|---|
| Developmental stage | 5, 24, 48, 72, 96, 120 hpf 1 9 and 14 dpf Adults | 7, 26, 50, 74, 98, 122 hpf 9 and 14 dpf |
| Samples | Microsomes from whole embryos/larvae/adults | Intact embryos and larvae |
| Substrate CYP activity | BOMR | BOMR |
| Negative control/Blank | Supersomes | Embryos/larvae in fish medium |
| Positive control | Adult zebrafish liver microsomes 1 | Embryos/larvae incubated with ER 2 |
| Biological replicates | Three (5–120 hpf; 14 dpf; adults) Two (9 dpf) | Three/developmental stage |
| Technical replicates | Two for 5–120 hpf Six for 9 and 14 dpf Three for adults | Two |
| Detection of resorufin formation | Microplate reader | Fluorescence microscope |
| Time Point | Hpf | Dpf | Number of Organisms/Biological Replicate |
|---|---|---|---|
| 1 | 1.5 | 0.06 | 30 |
| 2 | 6 | 0.25 | 30 |
| 3 | 14 | 0.58 | 30 |
| 4 | 24 | 1 | 20 |
| 5 | 36 | 1.5 | 20 |
| 6 | 48 | 2 | 20 |
| 7 | 60 | 2.5 | 20 |
| 8 | 72 | 3 | 20 |
| 9 | 84 | 3.5 | 20 |
| 10 | 96 | 4 | 10 |
| 11 | 120 | 5 | 10 |
| 12 | 144 | 6 | 10 |
| 13 | 192 | 8 | 10 |
| 14 | 240 | 10 | 10 |
| 15 | 288 | 12 | 10 |
| 16 | 336 | 14 | 10 |
| 17 | 384 | 16 | 10 |
| 18 | 432 | 18 | 10 |
| 19 | 480 | 20 | 10 |
| 20 | 528 | 22 | 10 |
| 21 | 576 | 24 | 10 |
| 22 | 624 | 26 | 10 |
| 23 | 672 | 28 | 10 |
| 24 | 720 | 30 | 10 |
| 25 | 768 | 32 | 10 |
| Gene | Sequence (5′ to 3′) | Accession Number | Reference |
|---|---|---|---|
| Target Genes | |||
| CYP1A | FW: GCATTACGATACGTTCGATAAGGAC RV: GCTCCGAATAGGTCATTGACGAT | NM_131879.1 | Goldstone et al., 2010 [22] |
| CYP1B1 | FW: GAGCACCGAAAGACCATTTCA RV: ATGGTCGGTGGCACAAACTC | NM_001045256.1 NM_001145708.1 | Olsvik et al., 2014 [93] |
| CYP1C1 | FW: AGTGGCACAGTCTACTTTGAGAG RV: TCGTCCATCAGCACTCAG | NM_001020610.2 | Goldstone et al., 2010 [22] |
| CYP1C2 | FW: GTGGTGGAGCACAGACTAAG RV: TTCAGTATGAGCCTCAGTCAAAC | NM_001114849.1 | Jönsson et al., 2007 [72] |
| CYP2K6 | FW: CCAGCTTTGTCCCTGTTTCTT RV: GCAGAGAGTTCAGCCTGTGAT | NM_200509.2 | Designed in-house |
| CYP3A65 | FW: CTTCGGCACCATGCTGAGAT RV: AGATACCCCAGATCCGTCCATA | NM_001037438.1 | Chang et al., 2013 [94] |
| CYP3C1 | FW: TCCAGACCTCTGGGAGTCTCCTAAT RV: GCATGAAGGCACACTGGTTGATCT | NM_212673.1 | Shaya et al., 2014 [61] |
| SULT1ST1 | FW: GTTCCTTCTTGGGTTTGTCT RV: CTGGCAGAGTGGAATAGTTG | NM_182941.1 | Liu et al., 2011 [95] |
| UGT1A1 | FW: TCCTTTGCCGCAGCATGTAT RV: ACTCTCTGGCTTTGGCTTCG | NM_001037428.2 | Wang et al., 2014 [96] |
| abcb4 | FW: TACTGATGATGCTTGGCTTAATC RV: TCTCTGGAAAGGTGAAGTTAGG | NM_001316714.1 NM_001114583.2 | Fischer et al., 2013 [53] |
| Reference Genes | |||
| 18S | FW: CGGAGAGGGAGCCTGAGAA RV: AGTCGGGAGTGGGTAATTTGC | Biga et al., 2005 [97] | |
| actb1 | FW: AAGTGCGACGTGGACA RV: GTTTAGGTTGGTCGTTCGTTTGA | NM_131031 | Gonzalez et al., 2006 [98] |
| hprt1 | FW: GTGGCTCTATGTGTGCT RV: CCTCCACAATCAAGACG | NM_212986.1 | Bio- Engineering Com.(Shanghai, China) |
| rpn2 | FW: TTGAGTTCAGCCAGCGT RV: TGGCAACAAATCGGCG | NM_212748.1 | De Wit et al., 2008 [99] |
| ef1a | FW: TGTCCTCAAGCCTGGTAT RV: CATTACCACGACGGATGT | NM_131263 | Houbrechts et al., 2016 [100] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbueken, E.; Bars, C.; Ball, J.S.; Periz-Stanacev, J.; Marei, W.F.A.; Tochwin, A.; Gabriëls, I.J.; Michiels, E.D.G.; Stinckens, E.; Vergauwen, L.; et al. From mRNA Expression of Drug Disposition Genes to In Vivo Assessment of CYP-Mediated Biotransformation during Zebrafish Embryonic and Larval Development. Int. J. Mol. Sci. 2018, 19, 3976. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123976
Verbueken E, Bars C, Ball JS, Periz-Stanacev J, Marei WFA, Tochwin A, Gabriëls IJ, Michiels EDG, Stinckens E, Vergauwen L, et al. From mRNA Expression of Drug Disposition Genes to In Vivo Assessment of CYP-Mediated Biotransformation during Zebrafish Embryonic and Larval Development. International Journal of Molecular Sciences. 2018; 19(12):3976. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123976
Chicago/Turabian StyleVerbueken, Evy, Chloé Bars, Jonathan S. Ball, Jelena Periz-Stanacev, Waleed F. A. Marei, Anna Tochwin, Isabelle J. Gabriëls, Ellen D. G. Michiels, Evelyn Stinckens, Lucia Vergauwen, and et al. 2018. "From mRNA Expression of Drug Disposition Genes to In Vivo Assessment of CYP-Mediated Biotransformation during Zebrafish Embryonic and Larval Development" International Journal of Molecular Sciences 19, no. 12: 3976. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19123976