Wound-Healing Markers Revealed by Proximity Extension Assay in Tears of Patients following Glaucoma Surgery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Tear Sample Analysis by PEA
2.2. Examination of Protein-Level Changes after Trabeculectomy
3. Materials and Methods
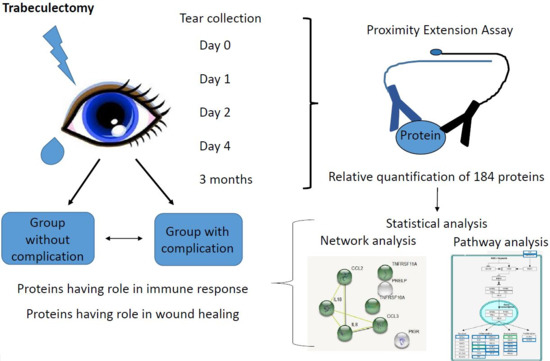
3.1. Subjects and Tear Sample Collection
3.2. Relative Quantification of 186 Proteins in Tear by Proximity Extension Assay
3.3. Functional Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tham, Y.-C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.-Y. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040: A Systematic Review and Meta-Analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Goldberg, J.L. Glaucoma 2.0: Neuroprotection, neuroregeneration, neuroenhancement. Ophthalmology 2012, 119, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Križaj, D.; Ryskamp, D.A.; Tian, N.; Tezel, G.; Mitchell, C.H.; Slepak, V.Z.; Shestopalov, V.I. From mechanosensitivity to inflammatory responses: New players in the pathology of glaucoma. Curr. Eye Res. 2014, 39, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Van Bergen, T.; Van de Velde, S.; Vandewalle, E.; Moons, L.; Stalmans, I. Improving patient outcomes following glaucoma surgery: State of the art and future perspectives. Clin. Ophthalmol. 2014, 8, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Bar-David, L.; Blumenthal, E.Z. Evolution of Glaucoma Surgery in the Last 25 Years. Rambam Maimonides Med. J. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-healing studies in cornea and skin: Parallels, differences and opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef] [PubMed]
- Ashby, B.D.; Garrett, Q.; Willcox, M.D. Corneal Injuries and Wound Healing – Review of Processes and Therapies. Austin J. Clin. Ophthalmol. 2014, 1, 1–25. [Google Scholar] [CrossRef]
- Agrawal, V.B.; Tsai, R.J.F. Importance of Corneal Epithelial. Indian J. Ophthalmol. 2003, 51, 5–15. [Google Scholar] [PubMed]
- Eslani, M.; Movahedan, A.; Afsharkhamseh, N.; Sroussi, H.; Djalilian, A.R. The Role of Toll-Like Receptor 4 in Corneal Epithelial Wound Healing. Investig. Opthalmol. Vis. Sci. 2014, 55, 6108. [Google Scholar] [CrossRef] [PubMed]
- Ljubimov, A.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2016, 49, 17–45. [Google Scholar] [CrossRef] [PubMed]
- Maycock, N.J.R.; Marshall, J. Genomics of corneal wound healing: A review of the literature. Acta Ophthalmol. 2014, 92, E170–E184. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.S.X.; Yin, J.; Xu, K.; Huang, J. Growth factors and corneal epithelial wound healing. Brain Res. Bull. 2010, 81, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West-Mays, J.A.; Dwivedi, D.J. The keratocyte: Corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006, 38, 1625–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, F.L.; Chaurasia, S.S.; Cutler, A.; Asosingh, K.; Kaur, H.; de Medeiros, F.W.; Agrawal, V.; Wilson, S.E. Corneal myofibroblast generation from bone marrow-derived cells. Exp. Eye Res. 2010, 91, 92–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, S.E.; Liu, J.J.; Mohan, R.R. Stromal-epithelial interactions in the cornea. Prog. Retin. Eye Res. 1999, 18, 293–309. [Google Scholar] [CrossRef]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrósio, R.; Hong, J.; Lee, J. The Corneal Wound Healing Response: Cytokine-mediated Interaction of the Epithelium, Stroma, and Inflammatory Cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef]
- Spadea, L.; Giammaria, D.; Trabucco, P. Corneal wound healing after laser vision correction. Br. J. Ophthalmol. 2016, 100, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Olayanju, J.A.; Hassan, M.B.; Hodge, D.O.; Khanna, C.L. Trabeculectomy-Related Complications in Olmsted County, Minnesota, 1985 Through 2010. JAMA Ophthalmol. 2015, 133, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Csutak, A.; Tőzsér, J.; Békési, L.; Hassan, Z.; Berta, A.; Silver, D.M. Plasminogen activator activity in tears after excimer laser photorefractive keratectomy. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3743–3747. [Google Scholar]
- Kallo, G.; Chatterjee, A.; Toth, M.; Rajnavölgyi, É.; Csutak, A.; Tozser, J.; Csősz, É. Relative quantification of human β-defensins by a proteomics approach based on selected reaction monitoring. Rapid Commun. Mass Spectrom. 2015, 29, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, A.; Rzepecka, A.; Dabrosin, C. Equal pro-inflammatory profiles of CCLs, CXCLs, and matrix metalloproteinases in the extracellular microenvironment in vivo in human dense breast tissue and breast cancer. Front. Immunol. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Carlsson, L.; Lind, A.-L.; Gordh, T.; Bodolea, C.; Kamali-Moghaddam, M.; Thulin, M. The body mass index (BMI) is significantly correlated with levels of cytokines and chemokines in cerebrospinal fluid. Cytokine 2015, 76, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Björkesten, J.; Enroth, S.; Shen, Q.; Wik, L.; Hougaard, D.M.; Cohen, A.S.; Sörensen, L.; Giedraitis, V.; Ingelsson, M.; Larsson, A.; et al. Stability of Proteins in Dried Blood Spot Biobanks. Mol. Cell. Proteomic 2017, 16, 1286–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillered, L.; Dahlin, A.P.; Clausen, F.; Chu, J.; Bergquist, J.; Hjort, K.; Enblad, P.; Lewén, A. Cerebral microdialysis for protein biomarker monitoring in the neurointensive care setting—A technical approach. Front. Neurol. 2014, 5, 245. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, M.; Viken, K.; Wang, Q.; Jagtap, P.; Bitterman, P.; Ingbar, D.; Wendt, C. Bronchoalveolar Lavage Fluid Protein Expression in Acute Respiratory Distress Syndrome Provides Insights into Pathways Activated in Subjects with Different Outcomes. Sci. Rep. 2017, 7, 7464. [Google Scholar] [CrossRef] [PubMed]
- Csosz, É.; Kalló, G.; Jakob, B.M.; Deák, E.; Csutak, A.; Tozsér, J. Quantitative body fluid proteomics in medicine—A focus on minimal invasiveness. J. Proteom. 2016, 153, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W. The power of tears: How tear proteomics research could revolutionize the clinic. Expert Rev. Proteom. 2017, 14, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, N.; Van Grasdorff, S.; Wouters, K.; Rozema, J.; Koppen, C.; Lion, E.; Cools, N.; Berneman, Z.; Tassignon, M.J. Human tears reveal insights into corneal neovascularization. PLoS ONE 2012, 7, e36451. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef] [PubMed]
- Roversi, P.; Marti, L.; Caputo, A.T.; Alonzi, D.S.; Hill, J.C.; Dent, K.C.; Kumar, A.; Levasseur, M.D.; Lia, A.; Waksman, T.; et al. Interdomain conformational flexibility underpins the activity of UGGT, the eukaryotic glycoprotein secretion checkpoint. Proc. Natl. Acad. Sci. USA 2017, 114, 8544–8549. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, S.; Ide, S.; Tokuyama, R.; Umeki, H.; Tatehara, S.; Kataoka, S.; Satomura, K. Leptin promotes wound healing in the skin. PLoS ONE 2015, 10, e0121242. [Google Scholar] [CrossRef] [PubMed]
- Kook, H.; Itoh, H.; Choi, B.S.; Sawada, N.; Doi, K.; Hwang, T.J.; Kim, K.K.; Arai, H.; Baik, Y.H.; Nakao, K.; et al. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1388–H1397. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Kwon, Y.W.; Park, G.T.; Do, E.K.; Yoon, J.W.; Kim, S.-C.; Ko, H.C.; Kim, M.B.; Kim, J.H. Atrial natriuretic peptide accelerates human endothelial progenitor cell-stimulated cutaneous wound healing and angiogenesis. Wound Repair Regen. 2018, 26, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernasconi, R.; Nystrom, A. Balance and circumstance: The renin angiotensin system in wound healing and fibrosis. Cell Signal. 2018, 51, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E.; Cleutjens, K.B.J.M.; Heeneman, S.; Koteliansky, V.E.; Burkly, L.C.; Daemen, M.J.A.P. Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype. Proc. Natl. Acad. Sci. USA 2000, 97, 7464–7469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, A.K.; Kisucka, J.; Brill, A.; Walsh, M.T.; Scheiflinger, F.; Wagner, D.D. ADAMTS13: A new link between thrombosis and inflammation. J. Exp. Med. 2008, 205, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Dhall, S.; Karim, Z.A.; Khasawneh, F.T.; Martins-Green, M. Platelet Hyperactivity in TNFSF14/LIGHT Knockout Mouse Model of Impaired Healing. Adv Wound Care 2016, 5, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.; Zahner, S.P.; Kim, G.; Shaikh, R.B.; Steinberg, M.W. The Tumor Necrosis Factor Family Member TNFSF14 (LIGHT) is Required for Resolution of Intestinal Inflammation in Mice. Gastroenterology 2015, 146, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Fang, D.; Fang, J.; Ren, X.; Yang, X.; Wen, F.; Su, S.B. Impaired Wound Healing with Defective Expression of Chemokines and Recruitment of Myeloid Cells in TLR3-Deficient Mice. J. Immunol. 2011, 186, 3710–3717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiang, J.G.; Zhai, M.; Liao, P.J.; Ho, C.; Gorbunov, N.V.; Elliott, T.B. Thrombopoietin Receptor Agonist Mitigates Hematopoietic Radiation Syndrome and Improves Survival after Whole-Body Ionizing Irradiation Followed by Wound Trauma. Mediat. Inflamm. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.E.; Stober, V.; Sobhany, M.; Zhuo, L.; Roberts, J.D.; Negishi, M.; Kimata, K.; Garantziotis, S. Inter-α-trypsin inhibitor promotes bronchial epithelial repair after injury through vitronectin binding. J. Biol. Chem. 2009, 284, 16922–16930. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.; Aaltonen, M.; Pan, P.; Vahatupa, M.; Kaipiainen, P.; May, U.; Prince, S.; Uusitalo-Järvinen, H.; Waheed, A.; Pastoreková, S. Role of carbonic anhydrases in skin wound healing. Exp. Mol. Med. 2017, 49, E334. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Carrozzi, V.; Sambandam, A.; Luis, E.; Lin, Z.; Jeet, S.; Lesch, J.; Hackney, J.; Kim, J.; Zhou, M.; Lai, J.; et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011, 12, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Johnston, A.; Fritz, Y.; Dawes, S.M.; Diaconu, D.; Al-Attar, P.M.; Guzman, A.M.; Chen, C.S.; Fu, W.; Gudjonsson, J.E.; McCormick, T.S.; et al. McCormick and NLW. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J. Immunol. 2013, 190, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Magadi, S.; Li, Z.; Smith, C.W.; Burns, A.R. IL-20 promotes epithelial healing of the injured mouse cornea. Exp. Eye Res. 2017, 154, 22–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sziksz, E.; Pap, D.; Lippai, R.; Béres, N.J.; Fekete, A.; Szabó, A.J.; Vannay, Á. Fibrosis Related Inflammatory Mediators: Role of the IL-10 Cytokine Family. Mediat. Inflamm. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Papanastasiou, A.D.; Sirinian, C.; Kalofonos, H.P. Identification of novel human receptor activator of nuclear factor-kB isoforms generated through alternative splicing: Implications in breast cancer cell survival and migration. Breast Cancer Res. 2012, 14, R112. [Google Scholar] [CrossRef] [PubMed]
- Hui, Q.; Jin, Z.; Li, X.; Liu, C.; Wang, X. FGF Family: From Drug Development to Clinical Application. Int. J. Mol. Sci. 2018, 19, 1875. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Yang, F.; Du, H.; Li, X.; Liu, J.; Dong, M.; Xu, X. Role of artemin in non-small cell lung cancer. Thorac. Cancer 2018, 9, 555–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrow, A.D.; Palarasah, Y.; Bugatti, M.; Holehouse, A.S.; Byers, D.E.; Holtzman, M.J.; Vermi, W.; Skjødt, K.; Crouch, E.; Colonna, M. OSCAR is a receptor for surfactant protein D that activates TNF-α release from human CCR2+ inflammatory monocytes. J. Immunol. 2015, 194, 3317–3326. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Dohi, T.; Maan, Z.N.; Rustad, K.C.; Kwon, S.H.; Padmanabhan, J.; Whittam, A.J.; Suga, H.; Duscher, D.; Rodrigues, M.; et al. Age-associated intracellular superoxide dismutase deficiency potentiates dermal fibroblast dysfunction during wound healing. Exp. Dermatol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.H.; Theilen, T.-M.; Masania, J.; Wunderle, M.; Karimi, J.; Vittas, S.; Bernauer, R.; Bierhaus, A.; Rabbani, N.; Thornalley, P.J.; et al. Aging-dependent reduction in glyoxalase 1 delays wound healing. Gerontology 2013, 59, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.; Nawroth, P.P. Reactive metabolites as a cause of late diabetic complications. Biochem. Soc. Trans. 2014, 42, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Treiber, N.; Maity, P.; Singh, K.; Ferchiu, F.; Wlaschek, M.; Scharffetter-Kochanek, K. The role of manganese superoxide dismutase in skin aging. Dermatoendocrinol 2012, 4, 232–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaniolo, K.; Gingras, M.-E.; Audette, M.; Guerin, S.L. Expression of the gene encoding poly(ADP-ribose) polymerase-1 is modulated by fibronectin during corneal wound healing. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4199–4210. [Google Scholar] [CrossRef] [PubMed]
- El-Hamoly, T.; Hegedus, C.; Lakatos, P.; Kovacs, K.; Bai, P.; El-Ghazaly, M.A.; El-Denshary, E.S.; Szabó, É.; Virág, L. Activation of poly(ADP-ribose) polymerase-1 delays wound healing by regulating keratinocyte migration and production of inflammatory mediators. Mol. Med. 2014, 20, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Gurtner, G. Black, White, and Gray: Macrophages in Skin Repair and Disease. Curr. Pathobiol. Rep. 2017, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, Y.; Luan, J.; Zhang, X.; Li, C.; Zhou, X.; Shi, L.; Wang, H.; Han, J. PRELP (proline/arginine-rich end leucine-rich repeat protein) promotes osteoblastic differentiation of preosteoblastic MC3T3-E1 cells by regulating the β-catenin pathway. Biochem. Biophys. Res. Commun. 2016, 470, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.; Maltseva, I.; Clay, S.M.; Pan, P.; Gajjala, A.; Chan, M.F. Effects of MMP12 on cell motility and inflammation during corneal epithelial repair. Exp. Eye Res. 2017, 160, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Nakamura, M.; Mishima, H.; Otori, T. Interleukin 6 promotes epithelial migration by a fibronectin-dependent mechanism. J. Cell Physiol. 1992, 153, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, S.O.; Evans, S.S.; Baumann, H.; Owczarczak, B.; Maier, P.; Vaughan, L.; Wang, W.C.; Unger, E.; Henderson, B.W. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer 2003, 88, 1772–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berta, A. Collection of tear samples with or without stimulation. Am. J. Ophthalmol. 1983, 96, 115–116. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive methode for the quantitation of microgram quantites of proiteins utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Saldanha, A.J. Java Treeview-extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y.; Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef] [PubMed]






| Protein Code According to String | Protein Code According to Olink | % of Samples in Group with No Complications | % of Samples in Group with Complications | Change of Protein Abundance | Biological Function | Role in Wound Healing |
|---|---|---|---|---|---|---|
| CD40LG | CD40-L | 74 | 45 | decrease | cytokine–cytokine receptor interaction, receptor binding * | lack of CD40L leads to excessive collagen deposition |
| ADAMTS13 | ADAM-TS13 | 68 | 45 | decrease | receptor binding * | lack of ADAMTS13 increases the extravasation of neutrophils |
| GIF | GIF | 68 | 45 | decrease | cyanocobalamin absorption # | not identified yet |
| FGF23 | FGF-23 | 89 | 55 | decrease | regulation of immune system process, regulation of MAPK cascade * | not FGF23 but other FGFs facilitate wound healing |
| CD84 | CD84 | 74 | 27 | decrease | modulation of the activation and differentiation of immune cells, regulation and interconnection of innate and adaptive immune response # | not identified yet |
| REN | REN | 84 | 55 | decrease | regulation of MAPK cascade, receptor binding * | depending on the conditions activates or inhibits wound healing |
| DECR1 | DECR1 | 95 | 73 | decrease | Metabolism # | not identified yet |
| AMBP | AMBP | 84 | 64 | decrease | regulation of immune system process, regulation of MAPK cascade * | AMBP deficiency is related to significantly more pathological-appearing cells in airway injury |
| FCGR2B | IgG Fc receptor II-b | 47 | 18 | decrease | regulation of immune system process * | not identified yet |
| THPO | THPO | 42 | 0 | decrease | regulation of immune system process, regulation of MAPK cascade, receptor binding * | THPO receptor agonist causes slight delay in wound healing |
| MARCO | MARCO | 42 | 9 | decrease | regulation of immune system process * | not identified yet |
| UGGT1 | GT | 26 | 0 | decrease | protein folding quality control # | not identified yet |
| NPPB | BNP | 47 | 18 | decrease | receptor binding * | promotes wound healing |
| LEP | LEP | 79 | 36 | decrease | regulation of immune system process, regulation of MAPK cascade, cytokine–cytokine receptor interaction * | promotes wound healing |
| CA5A | CA5A | 32 | 0 | decrease | Metabolism # | lower levels of carbonic anhydrase were detected in keloid scars |
| TNFSF14 | TNFSF14 | 100 | 73 | decrease | regulation of immune system process, cytokine–cytokine receptor interaction, receptor binding * | lack of TNFSF14 helps the accumulation of immune cells, increases cytokine levels, impairs wound healing |
| CCL3 | CCL3 | 89 | 64 | decrease | regulation of immune system process, regulation of mitogen-activated protein kinase cascade, cytokine–cytokine receptor interaction, receptor binding * | helps macrophage recruitment; decreased CCL3 levels were observed in impaired wound healing |
| IL17C | IL-17C | 37 | 64 | increase | cytokine–cytokine receptor interaction # | overexpression is observed in psoriasis |
| IL20RA | IL-20RA | 53 | 82 | increase | cytokine–cytokine receptor interaction * | promotes corneal epithelial healing |
| IL10RA | IL-10RA | 5 | 36 | increase | cytokine–cytokine receptor interaction * | promotes survival of myeloid cells |
| TNFSF11 | TRANCE | 16 | 36 | increase | cytokine–cytokine receptor interaction*, cell proliferation # | not identified yet |
| ARTN | ARTN | 47 | 91 | increase | neurotrophic factor, ligand for the RET receptor # | facilitates wound healing |
| FGF19 | FGF-19 | 37 | 64 | increase | regulation of bile acid synthesis # | not FGF19 but other FGFs facilitate wound healing |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csősz, É.; Tóth, N.; Deák, E.; Csutak, A.; Tőzsér, J. Wound-Healing Markers Revealed by Proximity Extension Assay in Tears of Patients following Glaucoma Surgery. Int. J. Mol. Sci. 2018, 19, 4096. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19124096
Csősz É, Tóth N, Deák E, Csutak A, Tőzsér J. Wound-Healing Markers Revealed by Proximity Extension Assay in Tears of Patients following Glaucoma Surgery. International Journal of Molecular Sciences. 2018; 19(12):4096. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19124096
Chicago/Turabian StyleCsősz, Éva, Noémi Tóth, Eszter Deák, Adrienne Csutak, and József Tőzsér. 2018. "Wound-Healing Markers Revealed by Proximity Extension Assay in Tears of Patients following Glaucoma Surgery" International Journal of Molecular Sciences 19, no. 12: 4096. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19124096