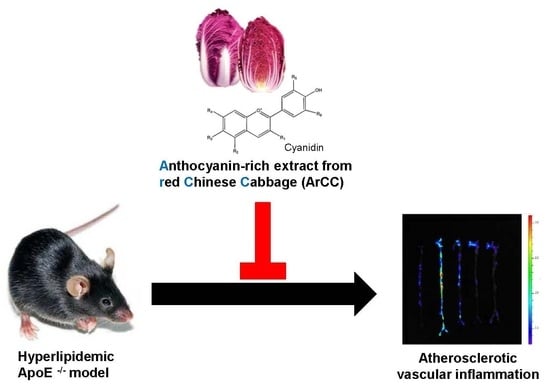
Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and Apo E−/− Mice
Abstract
:1. Introduction
2. Results
2.1. The Contents of the Anthocyanin-Rich Extract from Red Chinese Cabbage
2.2. ArCC Treatment Suppresses VCAM-1 Expression and Its Transcriptional Regulation in Tumor Necrosis Factor-α (TNF-α)-Stimulated Human Umbilical Vascular Endothelial Cells (HUVECs)
2.3. ArCC Treatment of TNF-α-Stimulated HUVECs Inhibited the Transcriptional Activity of NF-κB and Its Nuclear Localization
2.4. ArCC Treatment Inhibits Monocyte Adhesion on TNF-α-Stimulated HUVECs
2.5. Oral Administration of ArCC to Hyperlipidemic Apolipoprotein E-Deficient (ApoE−/−) Mice
2.6. ArCC Administration Regulates Lipid Profile Changes in Blood of ApoE−/− Mice
2.7. ArCC Administration Inhibits Vascular Inflammation of WD-Fed ApoE−/− Mice
2.8. ArCC Administration Inhibits Plaque Formation in the Aortas of ApoE−/− Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of ArCC
4.3. Monocyte-Endothelial Cell Adhesion Assay
4.4. Promoter Assay
4.5. Immunoblotting
4.6. Immunocytochemistry
4.7. Animal Experiments
4.8. Evaluation of Atherosclerotic Lesions
4.9. Plasma Analysis
4.10. Histological Staining
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ArCC | anthocyanin-rich extract from red Chinese cabbage |
| ApoE−/− | hyperlipidemic apolipoprotein E-deficient |
| C3G | cyanidin-3-glucoside |
| ApoE | apolipoprotein E |
| HUVECs | human umbilical vein endothelial cells |
| VCAM-1 | vascular cell adhesion molecule-1 |
| ICAM-1 | intercellular adhesion molecule-1 |
| TNF-α | tumor necrosis factor-alpha |
| MCP-1 | monocyte chemotactic protein-1 |
| NF-κB | nuclear factor κB |
| CAD | coronary artery disease |
References
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The Global Burden of Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008; p. 146. [Google Scholar]
- Beltrame, J.F. Epidemiology of coronary artery disease. In Coronary Artery Disease—Current Concepts in Epidemiology, Pathophysiology, Diagnostics and Treatment; Gaze, D., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Lichtman, A.H.; Binder, C.J.; Tsimikas, S.; Witztum, J.L. Adaptive immunity in atherogenesis: New insights and therapeutic approaches. J. Clin. Investig. 2013, 123, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; Heimler, D. HPLC-DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. Subsp. sylvestris L.). J. Agric. Food Chem. 2006, 54, 1342–1346. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.S.; Kim, H.K.; Lefeber, A.W.; Erkelens, C.; Choi, Y.H.; Verpoorte, R. Identification of phenylpropanoids in methyl jasmonate treated Brassica rapa leaves using two-dimensional nuclear magnetic resonance spectroscopy. J. Chromatogr. A 2006, 1112, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Rochfort, S.J.; Imsic, M.; Jones, R.; Trenerry, V.C.; Tomkins, B. Characterization of flavonol conjugates in immature leaves of pak choi [Brassica rapa L. Ssp chinensis L. (Hanelt.)] by HPLC-DAD and LC-MS/MS. J. Agric. Food Chem. 2006, 54, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, Q.H.; Xu, K. Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol. Sin. 2009, 30, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.; Kim, G.H. GC-MS Analysis of the Extracts from Korean Cabbage (Brassica campestris L. ssp. pekinensis) and Its Seed. Prev. Nutr. Food Sci. 2013, 18, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Schonhof, I.; Krumbein, A.; Bruckner, B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Nahrung 2004, 48, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; Di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super. Sanita 2007, 43, 394–405. [Google Scholar] [PubMed]
- Fuentes, F.; Paredes-Gonzalez, X.; Kong, A.T. Dietary Glucosinolates Sulforaphane, Phenethyl Isothiocyanate, Indole-3-Carbinol/3,3′-Diindolylmethane: Anti-Oxidative Stress/Inflammation, Nrf2, Epigenetics/Epigenomics and In Vivo Cancer Chemopreventive Efficacy. Curr. Pharmacol. Rep. 2015, 1, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary Intake of Carotenoids and Their Antioxidant and Anti-Inflammatory Effects in Cardiovascular Care. Mediat. Inflamm. 2013, 2013, 782137. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. Blueberries and Their Anthocyanins: Factors Affecting Biosynthesis and Properties. Compr. Rev. Food Sci. Food Saf. 2011, 10, 303–320. [Google Scholar] [CrossRef]
- Atalay, M.; Gordillo, G.; Roy, S.; Rovin, B.; Bagchi, D.; Bagchi, M.; Sen, C.K. Anti-angiogenic property of edible berry in a model of hemangioma. FEBS Lett. 2003, 544, 252–257. [Google Scholar] [CrossRef]
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Gonzalez-Paramas, A.M.; Santos-Buelga, C.; Mezzetti, B.; Quiles, J.L.; Battino, M.; Giampieri, F.; et al. Strawberry (cv. Romina) Methanolic Extract and Anthocyanin-Enriched Fraction Improve Lipid Profile and Antioxidant Status in HepG2 Cells. Int. J. Mol. Sci. 2017, 18, 1149. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of Berry polyphenols—Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar] [CrossRef]
- Kempe, S.; Kestler, H.; Lasar, A.; Wirth, T. NF-κB controls the global pro-inflammatory response in endothelial cells: Evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005, 33, 5308–5319. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.H.; Wei, Z.K.; Zhou, E.S.; Zhang, N.S.; Yang, Z.T. Cyanidin-3-O-β-glucoside inhibits lipopolysaccharide-induced inflammatory response in mouse mastitis model. J. Lipid Res. 2014, 55, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.J.; Liu, Y.; Wang, X.; Fan, Z.Q.; Chen, G.; Xu, M.; Bower, K.A.; Frank, J.A.; Ou, X.M.; Shi, X.L.; et al. Cyanidin-3-Glucoside Ameliorates Ethanol Neurotoxicity in the Developing Brain. J. Neurosci. Res. 2011, 89, 1676–1684. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Davies, T.S. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets. Cytokine Growth Factor Rev. 2015, 26, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Croce, G.; Tiberti, S.; Aggio, A.; Ferri, C. Flavonoids, Vascular Function and Cardiovascular Protection. Curr. Pharm. Des. 2009, 15, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lafuente, A.; Guillamon, E.; Villares, A.; Rostagno, M.A.; Martinez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Lockheart, M.S.; Steffen, L.M.; Rebnord, H.M.; Fimreite, R.L.; Ringstad, J.; Thelle, D.S.; Pedersen, J.I.; Jacobs, D.R., Jr. Dietary patterns, food groups and myocardial infarction: A case-control study. Br. J. Nutr. 2007, 98, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.J. Anthocyanins and heart health. Ann. Ist. Super. Sanita 2007, 43, 369–374. [Google Scholar] [PubMed]
- Wallace, T.C. Anthocyanins in Cardiovascular Disease. Adv. Nutr. 2011, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Paixao, J.; Nunes, C.; Dinis, T.C.; Almeida, L.M. Cyanidin-3-glucoside suppresses cytokine-induced inflammatory response in human intestinal cells: Comparison with 5-aminosalicylic acid. PLoS ONE 2013, 8, e73001. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Speciale, A.; Cristani, M.; Fratantonio, D.; Molonia, M.S.; Ranaldi, G.; Saija, A.; Cimino, F. Cyanidin-3-O-glucoside inhibits NF-κB signalling in intestinal epithelial cells exposed to TNF-α and exerts protective effects via Nrf2 pathway activation. Toxicol. Lett. 2016, 264, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Meskin, M.S.; Bidlack, W.R.; Davies, A.J.; Lewis, D.S.; Randolph, R.K. Phytochemicals: Mechanisms of Action; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- De Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passamonti, S.; Vrhovsek, U.; Vanzo, A.; Mattivi, F. The stomach as a site for anthocyanins absorption from food. FEBS Lett. 2003, 544, 210–213. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.K.; Choi, S.; Lee, Y.R.; Lee, E.O.; Park, M.S.; Lim, Y.P.; Park, J.T.; Jeon, B.H. Ethanol Extract of Brassica rapa ssp. pekinensis Suppresses Tumor Necrosis Factor-α-Induced Inflammatory Response in Human Umbilical Vein Endothelial Cells. J. Med. Food 2017, 20, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Lee, K.M.; Joo, H.K.; Lee, Y.R.; Park, M.S.; Kang, G.; Choi, S.; Lee, K.H.; Jeon, B.H. Ulmus davidiana ethanol extract inhibits monocyte adhesion to tumor necrosis factor-α-stimulated endothelial cells. Integr. Med. Res. 2016, 5, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kim, K.M.; Jeon, B.H.; Choi, J.W.; Choi, S. The n-butanol fraction of Naematoloma sublateritium suppresses the inflammatory response through downregulation of NF-κB in human endothelial cells. Int. J. Mol. Med. 2012, 29, 801–808. [Google Scholar] [PubMed]
- Jaffer, F.A.; Libby, P.; Weissleder, R. Molecular imaging of cardiovascular disease. Circulation 2007, 116, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tung, C.H.; Mahmood, U.; Ntziachristos, V.; Gyurko, R.; Fishman, M.C.; Huang, P.L.; Weissleder, R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation 2002, 105, 2766–2771. [Google Scholar] [CrossRef] [PubMed]








| Parameter | NC | WD | WD + Low-ArCC | WD + High-ArCC | WD + Statin |
|---|---|---|---|---|---|
| Start weight (g) | 26.1 ± 0.6 | 25.2 ± 0.5 | 26.4 ± 0.6 | 26.2 ± 0.5 | 25.9 ± 0.4 |
| Final weight (g) | 30.7 ± 0.7 | 34.3 ± 0.9 # | 33.8 ± 0.6 # | 32.0 ± 0.5 | 31.1 ± 0.3 |
| SBP (mmHg) | 108.3 ± 4.9 | 134.1 ± 3.6 # | 106.5 ± 8.6 * | 103.5 ± 8.8 * | 106.2 ± 7.4 * |
| DBP (mmHg) | 77.1 ± 9.7 | 88.2 ± 7.2 | 71.7 ± 6.8 | 71.3 ± 0.5 | 85.6 ± 7.3 |
| HR (bpm) | 320.8 ± 38.9 | 326.3 ± 26.2 | 331.3 ± 22.4 | 353.7 ± 42.6 | 325.1 ± 32.3 |
| Plasma Lipids | NC | WD | WD + Low-ArCC | WD + High-ArCC | WD + Statin |
|---|---|---|---|---|---|
| TC (mg/dL) | 361.8 ± 6.1 | 527.5 ± 9.7 # | 510.5 ± 6.9 | 466.6 ± 9.7 * | 395.9 ± 5.7 * |
| LDL + VLDL (mg/dL) | 316.9 ± 9.3 | 490.1 ± 5.1 # | 477.7 ± 5.4 * | 438.6 ± 8.9 * | 364.7 ± 8.3 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, H.K.; Choi, S.; Lee, Y.R.; Lee, E.O.; Park, M.S.; Park, K.B.; Kim, C.-S.; Lim, Y.P.; Park, J.-T.; Jeon, B.H. Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and Apo E−/− Mice. Int. J. Mol. Sci. 2018, 19, 816. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030816
Joo HK, Choi S, Lee YR, Lee EO, Park MS, Park KB, Kim C-S, Lim YP, Park J-T, Jeon BH. Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and Apo E−/− Mice. International Journal of Molecular Sciences. 2018; 19(3):816. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030816
Chicago/Turabian StyleJoo, Hee Kyoung, Sunga Choi, Yu Ran Lee, Eun Ok Lee, Myoung Soo Park, Kyu Been Park, Cuk-Seong Kim, Yong Pyo Lim, Jong-Tae Park, and Byeong Hwa Jeon. 2018. "Anthocyanin-Rich Extract from Red Chinese Cabbage Alleviates Vascular Inflammation in Endothelial Cells and Apo E−/− Mice" International Journal of Molecular Sciences 19, no. 3: 816. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19030816