A Lipophilic IR-780 Dye-Encapsulated Zwitterionic Polymer-Lipid Micellar Nanoparticle for Enhanced Photothermal Therapy and NIR-Based Fluorescence Imaging in a Cervical Tumor Mouse Model
Abstract
:1. Introduction
2. Results
2.1. Synthesis and Characterization of PCB-Lipid Micellar Nanoparticles
2.2. Synthesis and Characterization of PCB-Lipid–IR-780 Nanoparticles
2.3. Photothermal-Mediated Toxicity to NIR Laser-Irradiated TC-1 Cancer Cells Treated with PCB-Lipid–IR-780 Nanoparticles
2.4. Intracellular Uptake of PCB-Lipid–IR-780 Nanoparticles by TC-1 Cell Line
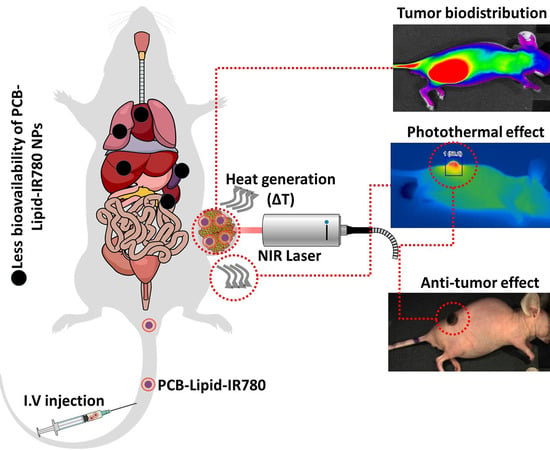
2.5. Biodistribution of PCB-Lipid–IR-780 Nanoparticles in TC-1 Xenograft Tumor Model
2.6. Antitumor Efficacy of PCB-Lipid–IR-780 Nanoparticles in TC-1 Xenograft Tumor Model after NIR Laser Irradiation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of CB-tBu Monomer
4.3. Synthesis of NHS-PCB-tBu via RAFT Polymerization
4.4. Synthesis of DPPE-PCB
4.5. Preparation and Characterization of PCB-Lipid–IR-780 Nanoparticles
4.6. Critical Micellar Concentration
4.7. Temperature Measurements under Near-IR Irradiation
4.8. Cell Culture and Animal Model
4.9. In Vitro Cell Viability Assay using MTS
4.10. Live and Dead Cell Assay
4.11. Confocal Microscope Imaging
4.12. In Vivo Near-IR Imaging and Biodistribution Analysis
4.13. In Vivo Photothermal Therapy
4.14. Statistically Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PTT | photothermal therapy |
| RAFT | reversible addition–fragmentation chain transfer |
| IR | infrared |
| NIR | near-infrared |
| ICG | indocyanine green |
| FDA | Food and Drug Administration |
| cRGD | cyclic Arg–Gly–Asp |
| EPR | enhanced permeability and retention |
| NMR | nuclear magnetic resonance |
| DPPE | 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine |
| TFA | trifluoroacetic acid |
| CLSM | confocal laser scanning microscopy |
| DAPI | 4′,6-diamidino-2-phenylindole, dihydrochloride |
| CMC | critical micellar concentration |
| RPMI | Roswell Park Memorial Institute medium |
| FBS | fetal bovine serum |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| SEM | standard error of the mean |
| ANOVA | analysis of variance |
| FOBI | fluorescence-labeled organism bioimaging instrument |
| PBS | phosphate-buffered saline |
| DMSO | dimethyl sulfoxide |
| DMF | dimethylformamide |
| TEM | transmission electron microscopy |
| AIBN | 2,2′-azodiisobutyronitrile |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DLS | Dynamic light scattering |
References
- Qasim, M.; Lim, D.J.; Park, H.; Na, D. Nanotechnology for diagnosis and treatment of infectious diseases. J. Nanosci. Nanotechnol. 2014, 14, 7374–7387. [Google Scholar] [CrossRef] [PubMed]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol. C 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Alves, C.G.; Lima-Sousa, R.; de Melo-Diogo, D.; Louro, R.O.; Correia, I.J. IR-780 based nanomaterials for cancer imaging and photothermal, photodynamic and combinatorial therapies. Int. J. Pharm. 2018, 542, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Liu, P.; Zheng, M.; Zhao, P.; Wang, Y.; Ma, Y.; Cai, L. IR-780 dye loaded tumor targeting theranostic nanoparticles for NIR imaging and photothermal therapy. Biomaterials 2013, 34, 6853–6861. [Google Scholar] [CrossRef] [PubMed]
- Palao-Suay, R.; Martin-Saavedra, F.M.; Rosa Aguilar, M.; Escudero-Duch, C.; Martin-Saldana, S.; Parra-Ruiz, F.J.; Rohner, N.A.; Thomas, S.N.; Vilaboa, N.; San Roman, J. Photothermal and photodynamic activity of polymeric nanoparticles based on alpha-tocopheryl succinate-RAFT block copolymers conjugated to IR-780. Acta Biomater. 2017, 57, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Qiu, X.; Tang, X.; Liu, W.; Wu, J.; Hu, Y. Self-assembled PEG–IR-780-C13 micelle as a targeting, safe and highly-effective photothermal agent for in vivo imaging and cancer therapy. Biomaterials 2015, 51, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhang, K.; Cao, Y.; Chen, X.; Wang, K.; Liu, M.; Pei, R. Hydrophobic IR-780 dye encapsulated in CRGD-conjugated solid lipid nanoparticles for NIR imaging-guided photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 12217–12226. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Hirn, S.; Semmler-Behnke, M.; Schleh, C.; Wenk, A.; Lipka, J.; Schaffler, M.; Takenaka, S.; Moller, W.; Schmid, G.; Simon, U.; et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur. J. Pharm. Biopharm. 2011, 77, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Wang, H.; Wang, Y.; Han, H.; Jin, Q.; Ji, J. IR-780 loaded phospholipid mimicking homopolymeric micelles for near-IR imaging and photothermal therapy of pancreatic cancer. ACS Appl. Mater. Interfaces 2016, 8, 6852–6858. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yu, Q.; Xue, H.; Cheng, G.; Jiang, S. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew. Chem. 2010, 49, 3771–3776. [Google Scholar] [CrossRef] [PubMed]
- Uthaman, S.; Mathew, A.P.; Park, H.J.; Lee, B.I.; Kim, H.S.; Huh, K.M.; Park, I.K. IR 780-loaded hyaluronic acid micelles for enhanced tumor-targeted photothermal therapy. Carbohydr. Polym. 2018, 181, 1–9. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hu, H.Y.; Tan, T.; Wang, H.; Sun, K.X.; Li, Y.P.; Zhang, Z.W. IR-780-loaded polymeric micelles enhance the efficacy of photothermal therapy in treating breast cancer lymphatic metastasis in mice. Acta Pharmacol. Sin. 2018, 39, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Cheng, H.; Yuan, A.; Tang, X.; Wu, J.; Hu, Y. Hydrophobic IR-780 encapsulated in biodegradable human serum albumin nanoparticles for photothermal and photodynamic therapy. Acta Biomater. 2015, 14, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Wang, H.; Zhang, A.; Zhao, C.; Chen, Y.; Li, X. Bright and stable near-infrared pluronic-silica nanoparticles as a contrast agent for in vivo optical imaging. J. Mater. Chem. B 2016, 4, 5560–5566. [Google Scholar] [CrossRef] [PubMed]
- Eggeling, C.; Widengren, J.; Rigler, R.; Seidel, C.A. Photobleaching of fluorescent dyes under conditions used for single-molecule detection: Evidence of two-step photolysis. Anal. Chem. 1998, 70, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Rustique, E.; Henry, M.; Guidetti, M.; Josserand, V.; Sancey, L.; Boutet, J.; Coll, J.L. Targeting tumors with cyclic RGD-conjugated lipid nanoparticles loaded with an IR-780 NIR dye: In vitro and in vivo evaluation. Int. J. Pharm. 2017, 532, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, K.; Yang, X.; Zhou, Y.; Ping, Q.; Oupicky, D.; Sun, M. Dual-function nanostructured lipid carriers to deliver IR-780 for breast cancer treatment: Anti-metastatic and photothermal anti-tumor therapy. Acta Biomater. 2017, 53, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Duan, W.; Li, Y.; Wu, H.; Zhou, Y.; Pan, M.; Liu, H.; Liu, X.; Zheng, H. NIR-laser-controlled drug release from DOX/IR-780-loaded temperature-sensitive-liposomes for chemo-photothermal synergistic tumor therapy. Theranostics 2016, 6, 2337–2351. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, S.; Zhang, T.; Wan, G.; Chen, B.; Xiong, Q.; Zhang, J.; Zhang, W.; Wang, Y. Pullulan-coated phospholipid and pluronic F68 complex nanoparticles for carrying IR-780 and paclitaxel to treat hepatocellular carcinoma by combining photothermal therapy/photodynamic therapy and chemotherapy. Int. J. Nanomed. 2017, 12, 8649–8670. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yu, M.; Wang, J.P.; Tan, F.P.; Li, N. The mitochondria-targeted and IR-780-regulated theranosomes for imaging and enhanced photodynamic/photothermal therapy. RSC Adv. 2016, 6, 11070–11076. [Google Scholar] [CrossRef]
- Li, W.; Peng, J.; Yang, Q.; Chen, L.; Zhang, L.; Chen, X.; Qian, Z. Alpha-lipoic acid stabilized DTX/IR-780 micelles for photoacoustic/fluorescence imaging guided photothermal therapy/chemotherapy of breast cancer. Biomater. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Yan, F.; Wang, F.; Qin, W.; Wu, G.; Yang, X.; Shao, C.; Chung, L.W.; Yuan, J. IR-780 dye for near-infrared fluorescence imaging in prostate cancer. Med. Sci. Monit. 2015, 21, 511–517. [Google Scholar] [PubMed]
- Lepock, J.R. Cellular effects of hyperthermia: Relevance to the minimum dose for thermal damage. Int. J. Hyperther. 2003, 19, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Mondini, S.; Leonzino, M.; Drago, C.; Ferretti, A.M.; Usseglio, S.; Maggioni, D.; Tornese, P.; Chini, B.; Ponti, A. Zwitterion-coated iron oxide nanoparticles: Surface chemistry and intracellular uptake by hepatocarcinoma (HepG2) cells. Langmuir 2015, 31, 7381–7390. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.M.; Lash, M.H.; Sparks, S.M.; Uhrich, K.E. Preferential cellular uptake of amphiphilic macromolecule-lipid complexes with enhanced stability and biocompatibility. J. Control. Release 2011, 153, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid-polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Miranda, O.R.; Moyano, D.F.; Walden, C.A.; Giri, K.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Reid, J.M.; Mukherjee, P. Modulating pharmacokinetics, tumor uptake and biodistribution by engineered nanoparticles. PLoS ONE 2011, 6, e24374. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, L.; Jiang, S. Superhydrophilic zwitterionic polymers stabilize liposomes. Langmuir 2012, 28, 11625–11632. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendrakumar, S.K.; Chang, N.-C.; Mohapatra, A.; Uthaman, S.; Lee, B.-I.; Tsai, W.-b.; Park, I.-K. A Lipophilic IR-780 Dye-Encapsulated Zwitterionic Polymer-Lipid Micellar Nanoparticle for Enhanced Photothermal Therapy and NIR-Based Fluorescence Imaging in a Cervical Tumor Mouse Model. Int. J. Mol. Sci. 2018, 19, 1189. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19041189
Rajendrakumar SK, Chang N-C, Mohapatra A, Uthaman S, Lee B-I, Tsai W-b, Park I-K. A Lipophilic IR-780 Dye-Encapsulated Zwitterionic Polymer-Lipid Micellar Nanoparticle for Enhanced Photothermal Therapy and NIR-Based Fluorescence Imaging in a Cervical Tumor Mouse Model. International Journal of Molecular Sciences. 2018; 19(4):1189. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19041189
Chicago/Turabian StyleRajendrakumar, Santhosh Kalash, Ning-Chu Chang, Adityanarayan Mohapatra, Saji Uthaman, Byeong-Il Lee, Wei-bor Tsai, and In-Kyu Park. 2018. "A Lipophilic IR-780 Dye-Encapsulated Zwitterionic Polymer-Lipid Micellar Nanoparticle for Enhanced Photothermal Therapy and NIR-Based Fluorescence Imaging in a Cervical Tumor Mouse Model" International Journal of Molecular Sciences 19, no. 4: 1189. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19041189