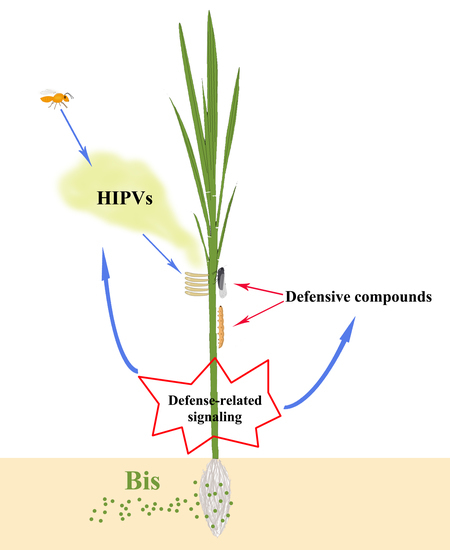
The Commonly Used Bactericide Bismerthiazol Promotes Rice Defenses against Herbivores
Abstract
:1. Introduction
2. Results
2.1. Bismerthiazol Treatment Enhances the Direct Resistance of Rice to WBPH and Slightly Impairs Plant Growth
2.2. Bismerthiazol Induces the Biosynthesis of Constitutive and/or Elicited JA, JA-Ile, ET and H2O2, but Not SA
2.3. Bismerthiazol Treatment Alters the Volatile Chemical Profile of Rice
2.4. Bismerthiazol Treatment Enhances the Indirect Resistance of Rice to WBPH
2.5. Bismerthiazol Treatment Also Enhances the Resistance of Rice to BPH and SSB
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Compounds
4.3. Insects
4.4. Plant Treatments
4.5. Measurement of Plant Growth Parameters
4.6. qRT-PCR
4.7. WBPH and BPH Performance Measurement
4.8. SSB Performance Measurement
4.9. JA, JA-Ile, SA, ET and H2O2 Analysis
4.10. Collection, Isolation and Identification of Volatile Compounds
4.11. Field Experiment
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| JA | Jasmonic acid |
| JA-Ile | Jasmonoyl-isoleucine conjugate |
| SA | Salicylic acid |
| ET | Ethylene |
| BTH | Benzo (1,2,3) thiadiazole-7-carbothioic acid S-methyl ester |
| MeJA | Methyl ester of JA |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| TrypPIs | Trypsin proteinase inhibitors |
| SSB | Striped stem borer |
| BPH | Brown planthopper |
| Xoo | Xanthomonas oryzae pv. Oryzae |
| WBPH | white-backed planthopper |
| MeSA | Methyl salicylate |
| SAMT | Salicylic acid carboxyl methyltransferase |
References
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Makita, S.; Schelbert, S.; Sano, S.; Ochiai, M.; Tsuchiya, T.; Hasegawa, S.F.; Hortensteiner, S.; Tanaka, A.; Tanaka, R. Reexamination of chlorophyllase function implies its involvement in defense against chewing herbivores. Plant Physiol. 2015, 167, 660–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ju, H.; Zhou, G.; Zhu, C.; Erb, M.; Wang, X.; Wang, P.; Lou, Y. An EAR-motif-containing ERF transcription factor affects herbivore-induced signaling, defense and resistance in rice. Plant J. 2011, 68, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Lu, J.; Li, J. Herbivore-Induced Defenses in Rice and Their Potential Application in Rice Planthopper Management. In Rice Planthoppers; Heong, K.L., Cheng, J., Escalada, M.M., Eds.; Zhejiang University Press: Hangzhou, China, 2015; pp. 91–116. [Google Scholar]
- Bektas, Y.; Eulgem, T. Synthetic plant defense elicitors. Front. Plant Sci. 2014, 5, 804. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Yu, Z.; Erb, M.; Turlings, T.C.; Wang, B.; Qi, J.; Liu, S.; Lou, Y. The broad-leaf herbicide 2,4-dichlorophenoxyacetic acid turns rice into a living trap for a major insect pest and a parasitic wasp. New Phytol. 2012, 194, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Beckers, G.J.; Conrath, U. Priming for stress resistance: From the lab to the field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef] [PubMed]
- LaMondia, J.A. Actigard Increases Fungicide Efficacy against Tobacco Blue Mold. Plant Dis. 2008, 92, 1463–1467. [Google Scholar] [CrossRef]
- Thaler, J.S.; Stout, M.J.; karban, R.; Duffey, S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996, 22, 1767–1781. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.F.; Redman, A.M. Age-dependent effects of jasmonic acid treatment and wind exposure on foliar oxidase activity and insect resistance in tomato. J. Chem. Ecol. 1999, 5, 271–281. [Google Scholar] [CrossRef]
- Thaler, J.S. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature 1999, 399, 686–688. [Google Scholar] [CrossRef]
- Pauwels, L.; Morreel, K.; Witte, E.D.; Lammertyn, F.; Montagu, M.V.; Boerjan, W.; Inzé, D.; Goossens, A. Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 2007, 105, 1380–1385. [Google Scholar] [CrossRef] [PubMed]
- Felton, G.W.; Duffey, S.S. Ascorbate Oxidation Reduction in Helicoverpa zea as a Scavenging System Against Dietary Oxidants. Arch. Insect Biochem. Physiol. 1992, 19, 29–37. [Google Scholar] [CrossRef]
- Stout, M.J.; Duffey, S.S. Characterization of induced resistance in tomato plants. Entomol. Exp. Appl. 1996, 79, 273–283. [Google Scholar] [CrossRef]
- Li, R.; Schuman, M.C.; Wang, Y.; Llorca, L.C.; Bing, J.; Bennion, A.; Halitschke, R.; Baldwin, I.T. Jasmonate signaling makes flowers attractive to pollinators and repellant to florivores in nature. J. Integr. Plant Biol. 2018, 60, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Kramell, R.; Atzorn, R.; Schneider, G.; Miersch, O.; Bruckner, C.; Schmidt, J.; Sembdner, G.; Parthier, B. Occurrence and Identification of Jasmonic Acid and Its Amino Acid Conjugates Induced by Osmotic Stress in Barley Leaf Tissue. J. Plant Growth Regul. 1995, 14, 29–36. [Google Scholar] [CrossRef]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aleman, G.H.; Machado, R.A.R.; Baldwin, I.T.; Boland, W. JA-Ile-macrolactones uncouple growth and defense in wild tobacco. Org. Biomol. Chem. 2017, 15, 3391–3395. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Campbell, C.A.M.; Chamberlain, K.; Guerrieri, E.; Hick, A.J.; Martin, J.L.; Matthes, M.; Napier, J.A.; Pettersson, J.; Pickett, J.A.; et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 9329–9334. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Ishiwari, H. Tiadinil, a plant activator of systemic acquired resistance, boosts the production of herbivore-induced plant volatiles that attract the predatory mite Neoseiulus womersleyi in the tea plant Camellia sinensis. Exp. Appl. Acarol. 2012, 58, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, I.S.; Erb, M.; Sarhan, A.A.; El-Husseini, M.M.; Mandour, N.S.; Turlings, T.C. Less is more: Treatment with BTH and laminarin reduces herbivore-induced volatile emissions in maize but increases parasitoid attraction. J. Chem. Ecol. 2012, 38, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, I.S.; Erb, M.; Turlings, T.C. Plant strengtheners enhance parasitoid attraction to herbivore-damaged cotton via qualitative and quantitative changes in induced volatiles. Pest Manag. Sci. 2015, 71, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhou, M.; Ye, Z. The action mode of saikuzuo against xanthomon as oryzae pv. oryzae. Acta Phytopathol. Sin. 1997, 27, 237–241. [Google Scholar]
- Shen, G.; Zhou, M. Resistance monitoring of Xanthomonas oryzae pv. axyzae to saikuzuo. Plant Prot. 2002, 28, 9–11. [Google Scholar]
- Huang, Q.; Zhou, M.; Ye, Z. Physiological characters of the resistant mutant of Xanthomonas citri to amicarthiazol. Acta Phytopathol. Sin. 2003, 33, 63–66. [Google Scholar]
- Liang, X.; Yu, X.; Dong, W.; Guo, S.; Xu, S.; Wang, J.; Zhou, M. Two thiadiazole compounds promote rice defence against Xanthomonas oryzae pv. oryzae by suppressing the bacterium’s production of extracellular polysaccharides. Mol. Plant Pathol. 2015, 16, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Stout, M.J.; Qian, Q.; Chen, F. Genetic, Molecular and Genomic Basis of Rice Defense against Insects. Crit. Rev. Plant Sci. 2012, 31, 74–91. [Google Scholar] [CrossRef]
- Lou, Y.G.; Ma, B.; Cheng, J.A. Attraction of the parasitoid Anagrus nilaparvatae to rice volatiles induced by the rice brown planthopper Nilaparvata lugens. J. Chem. Ecol. 2005, 31, 2357–2372. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.G.; Du, M.H.; Turlings, T.C.J.; Cheng, J.A.; Shan, W.F. Exogenous application of jasmonic acid induces volatile emissions in rice and enhances parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae. J. Chem. Ecol. 2005, 31, 1985–2002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Qi, J.; Ren, N.; Cheng, J.; Erb, M.; Mao, B.; Lou, Y. Silencing OsHI-LOX makes rice more susceptible to chewing herbivores, but enhances resistance to a phloem feeder. Plant J. 2009, 60, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Hu, L.; Zhang, T.; Zhang, G.; Lou, Y. OsMPK3 positively regulates the JA signaling pathway and plant resistance to a chewing herbivore in rice. Plant Cell Rep. 2013, 32, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, M.; Li, R.; Zhang, T.; Zhou, G.; Wang, Q.; Lu, J.; Lou, Y. The Rice Transcription Factor WRKY53 Suppresses Herbivore-Induced Defenses by Acting as a Negative Feedback Modulator of Mitogen-Activated Protein Kinase Activity. Plant Physiol. 2015, 169, 2907–2921. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Robert, C.A.; Riemann, M.; Cosme, M.; Mene-Saffrane, L.; Massana, J.; Stout, M.J.; Lou, Y.; Gershenzon, J.; Erb, M. Induced jasmonate signaling leads to contrasting effects on root damage and herbivore performance. Plant Physiol. 2015, 167, 1100–1116. [Google Scholar] [CrossRef] [PubMed]
- Mewis, I.; Appel, H.M.; Hom, A.; Raina, R.; Schultz, J.C. Major signaling pathways modulate Arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 2005, 138, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Delker, C.; Stenzel, I.; Hause, B.; Miersch, O.; Feussner, I.; Wasternack, C. Jasmonate biosynthesis in Arabidopsis thaliana—Enzymes, products, regulation. Plant Biol. 2006, 8, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Riemann, M.; Takano, M. Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling. Plant Cell Environ. 2008, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.; Ge, L.; Hu, L.; Li, J.; Han, X.; Zhang, T.; Lu, J.; et al. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Armstrong, C.M.; Zhou, M.; Duan, Y. Bismerthiazol Inhibits Xanthomonas citri subsp. citri Growth and Induces Differential Expression of Citrus Defense-Related Genes. Phytopathology 2016, 106, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Qi, J.; Zhu, X.; Mao, B.; Zeng, L.; Wang, B.; Li, Q.; Zhou, G.; Xu, X.; Lou, Y.; et al. The rice hydroperoxide lyase OsHPL3 functions in defense responses by modulating the oxylipin pathway. Plant J. 2012, 71, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.; Ju, H.; Liu, X.; Erb, M.; Wang, X.; Lou, Y. Contrasting effects of ethylene biosynthesis on induced plant resistance against a chewing and a piercing-sucking herbivore in rice. Mol. Plant 2014, 7, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, X.; Yan, F.; Wang, X.; Li, R.; Cheng, J.; Lou, Y. Genome-wide transcriptional changes and defence-related chemical profiling of rice in response to infestation by the rice striped stem borer Chilo suppressalis. Physiol. Plant 2011, 143, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, T.; Wang, W.; Cao, T.; Li, R.; Lou, Y. Silencing OsSLR1 enhances the resistance of rice to the brown planthopper Nilaparvata lugens. Plant Cell Environ. 2017, 40, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, J.; Han, X.; Li, R.; Wu, J.; Yu, H.; Hu, L.; Xiao, Y.; Lu, J.; Lou, Y. Jasmonic acid carboxyl methyltransferase regulates development and herbivory-induced defense response in rice. J. Integr. Plant Biol. 2016, 58, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, M.; Li, R.; Lou, Y. OsWRKY53, a versatile switch in regulating herbivore-induced defense responses in rice. Plant Signal. Behav. 2016, 11, e1169357. [Google Scholar] [CrossRef] [PubMed]
- Li, J. Comparison of Defense Responses in Rice Induced by Feeding and Oviposition of the Brown Planthopper Nilaparvata lugens and Their Underlying Mechanisms. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2015. [Google Scholar]
- Huangfu, J.; Li, J.; Li, R.; Ye, M.; Kuai, P.; Zhang, T.; Lou, Y. The Transcription Factor OsWRKY45 Negatively Modulates the Resistance of Rice to the Brown Planthopper Nilaparvata lugens. Int. J. Mol. Sci. 2016, 17, 697. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Baldwin, I.T. Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores. Plant Physiol. 2006, 140, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.W.; Wang, R.; Yang, T.; Jiang, L.; Zhang, Z.Z. Functional Characterization of Salicylic Acid Carboxyl Methyltransferase from Camellia sinensis, Providing the Aroma Compound of Methyl Salicylate during the Withering Process of White Tea. J. Agric. Food Chem. 2017, 65, 11036–11045. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.S.; Kollner, T.G.; Wiggins, G.; Grant, J.; Degenhardt, J.; Chen, F. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J. 2008, 55, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.; Vaughn, K.A.; et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, P. Screening and Primary Field Evaluation of Infochemicals Manipulating Behavioral Responses of Rice Brown Planthopper Nilaparvata lugens (Stål) and Its Egg Parasitoid Anagrus nilaparvatae. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2011. [Google Scholar]
- Ma, B.; Lou, Y.; Chen, J. Effects of some biotic factors on activities of the volatiles emitted from rice plants infested by the rice brown planthopper, Nilaparvata lugens (Stål). J. Zhejiang Univ. 2004, 30, 589–595. [Google Scholar]
- Wang, Q.; Xin, Z.; Li, J.; Hu, L.; Lou, Y.; Lu, J. (E)-β-caryophyllene functions as a host location signal for the rice white-backed planthopper Sogatella furcifera. Physiol. Mol. Plant Pathol. 2015, 91, 106–112. [Google Scholar] [CrossRef]
- Koo, Y.J.; Kim, M.A.; Kim, E.H.; Song, J.T.; Jung, C.; Moon, J.K.; Kim, J.H.; Seo, H.S.; Song, S.I.; Kim, J.K.; et al. Overexpression of salicylic acid carboxyl methyltransferase reduces salicylic acid-mediated pathogen resistance in Arabidopsis thaliana. Plant Mol. Biol. 2007, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Yu, X.; Pan, X.; Wu, J.; Duan, Y.; Wang, J.; Zhou, M. A thiadiazole reduces the virulence of Xanthomonas oryzae pv. oryzae by inhibiting the histidine utilization pathway and quorum sensing. Mol. Plant Pathol. 2016, 19, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Forno, D.A.; Cock, J.H.; Gomez, K.A. Laboratory Manual for Physiological Studies of Rice, 3rd ed.; International Rice Research Institute: Los Baños, Philippines, 1976. [Google Scholar]
- Fu, Q.; Zhang, Z.; Hu, C.; Lai, F.; Sun, Z. A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl. Entomol. Zool 2001, 36, 111–116. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Lou, Y.; Cheng, J. Role of ethylene signaling in the production of rice volatiles induced by the rice brown planthopper Nilaparvata lugens. Chin. Sci. Bull. 2006, 51, 2457–2465. [Google Scholar] [CrossRef]
- Qi, J.; Zhou, G.; Yang, L.; Erb, M.; Lu, Y.; Sun, X.; Cheng, J.; Lou, Y. The chloroplast-localized phospholipases D α4 and α5 regulate herbivore-induced direct and indirect defenses in rice. Plant Physiol. 2011, 157, 1987–1999. [Google Scholar] [CrossRef] [PubMed]






| No | Compound | Con | Bis | WBPH | Bis + WBPH |
|---|---|---|---|---|---|
| 1 | 2-Heptanone | 4.14 ± 1.59 b | 5.70 ± 1.69 b | 15.77 ± 1.23 a | 6.64 ± 0.85 b |
| 2 | 2-Heptanol | 0.49 ± 0.22 b | 0.50 ± 0.15 b | 4.13 ± 0.44 a | 0.59 ± 0.16 b |
| 3 | α-Thujene | - | 0.29 ± 0.17 a | 0.68 ± 0.17 a | 0.44 ± 0.04 a |
| 4 | α-Pinene | 0.53 ± 0.32 a | 0.94 ± 0.43 a | 0.81 ± 0.23 a | 0.71 ± 0.17 a |
| 5 | Myrcene | 1.20 ± 0.26 b | 2.12 ± 0.26 a | 1.72 ± 0.33 ab | 1.19 ± 0.26 b |
| 6 | (+)-Limonene | 0.86 ± 0.19 b | 1.69 ± 0.51 b | 11.95 ± 3.02 a | 10.70 ± 0.89 a |
| 7 | (E)-Linalool oxide | 0.14 ± 0.03 c | 0.32 ± 0.09 c | 2.98 ± 0.62 a | 0.93 ± 0.23 b |
| 8 | Linalool | 0.56 ± 0.07 c | 0.57 ± 0.23 c | 63.74 ± 15.76 a | 26.91 ± 4.48 b |
| 9 | Methyl salicylate | 0.15 ± 0.02 d | 0.41 ± 0.16 c | 6.05 ± 1.0 4 a | 2.06 ± 0.45 b |
| 10 | Unknown 1 | - | 1.02 ± 0.28 a | 1.34 ± 0.26 a | 0.54 ± 0.10 b |
| 11 | Unknown 2 | - | - | 1.28 ± 0.41 a | 0.33 ± 0.16 a |
| 12 | α-Copaene | - | - | 1.15 ± 0.37 a | 1.24 ± 0.04 a |
| 13 | Sesquithujene | - | 0.37 ± 0.10 b | 1.05 ± 0.21 a | 0.50 ± 0.02 b |
| 14 | α-Cedrene | 0.32 ± 0.04 a | 0.59 ± 0.14 a | 0.75 ± 0.16 a | 0.55 ± 0.06 a |
| 15 | (E)-β-Caryophyllene | 0.65 ± 0.07 b | 1.11 ± 0.39 b | 2.41 ± 0.46 a | 1.67 ± 0.14 ab |
| 16 | (E)-α-Bergamotene | - | 0.20 ± 0.08 b | 3.35 ± 0.92 a | 1.67 ± 0.10 a |
| 17 | Sesquisabinene A | - | - | 3.30 ± 0.87 a | 1.71 ± 0.12 b |
| 18 | (E)-β-Farnesene | - | - | 2.91 ± 0.83 a | 1.30 ± 0.10 a |
| 19 | α-Curcumene | - | 0.94 ± 0.34 a | 2.79 ± 0.81 a | 1.33 ± 0.16 a |
| 20 | Zingiberene | 1.13 ± 0.27 c | 1.41 ± 0.27 c | 10.33 ± 2.74 a | 5.08 ± 0.48 b |
| 21 | β-Bisabolene | 0.40 ± 0.11 c | - | 4.51 ± 1.27 a | 1.95 ± 0.20 b |
| 22 | β-Sesquiphellandrene | - | 0.48 ± 0.07 c | 7.70 ± 2.19 a | 3.72 ± 0.34 b |
| 23 | (E)-γ-Bisabolene | - | - | 3.78 ± 1.09 a | 2.09 ± 0.25 a |
| Total | 10.57 ± 3.17 c | 18.80 ± 5.36 c | 154.48 ± 35.44 b | 73.83 ± 9.78 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Mo, X.; Wang, W.; Chen, X.; Lou, Y. The Commonly Used Bactericide Bismerthiazol Promotes Rice Defenses against Herbivores. Int. J. Mol. Sci. 2018, 19, 1271. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051271
Zhou P, Mo X, Wang W, Chen X, Lou Y. The Commonly Used Bactericide Bismerthiazol Promotes Rice Defenses against Herbivores. International Journal of Molecular Sciences. 2018; 19(5):1271. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051271
Chicago/Turabian StyleZhou, Pengyong, Xiaochang Mo, Wanwan Wang, Xia Chen, and Yonggen Lou. 2018. "The Commonly Used Bactericide Bismerthiazol Promotes Rice Defenses against Herbivores" International Journal of Molecular Sciences 19, no. 5: 1271. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051271