Silver Nanoparticles: Two-Faced Neuronal Differentiation-Inducing Material in Neuroblastoma (SH-SY5Y) Cells
Abstract
:1. Introduction
2. Results
2.1. Influence of Exposure to AgNP or RA on SH-SY5Y Cell Viability and Differentiation
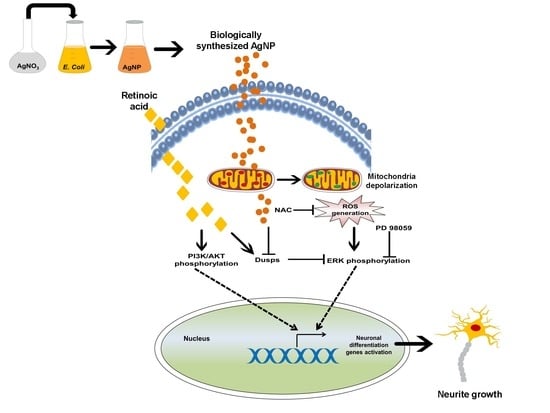
2.2. AgNP and RA Treatment Modulate DUSP Expression Levels and the Activation of Kinase Signaling
2.3. AgNP and RA Treatment Have Differential Effects on Intracellular ROS Generation, Mitochondrial Membrane Depolarization, and Antioxidant Gene Expression
2.4. A ROS Scavenging Agent and ERK and AKT Inhibitors Have Differential Effects on AgNP- and RA-Induced Neuronal Differentiation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. AgNP Synthesis and Characterization
4.3. SH-SY5Y Cell Culture and Treatment with AgNP and RA
4.4. Cytotoxicity Assays
4.5. Immunofluorescence Staining
4.6. Neurite Growth Analysis
4.7. Intracellular ROS Measurements and Quantification of Antioxidant Gene Expression
4.8. RNA Extraction, cDNA Synthesis, and mRNA Expression Analysis
4.9. ΔΨm Analysis Using JC-1
4.10. Western Blot Analysis
4.11. Statistical Analyses
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNP | silver nanoparticle |
| RA | all-trans-retinoic acid |
| ROS | reactive oxygen species |
| DUSP | dual-specificity phosphatase |
| NAC | N-acetyl-l-cysteine |
| ERK | extracellular-signal-regulated kinase |
| AKT | AK mouse strain transforming |
| PD | Parkinson’s disease |
| SH-SY5Y | human neuroblastoma cell line |
| GO | graphene oxide |
| TEM | transmission electron microscopy |
| DLS | dynamic light scattering |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPX | glutathione peroxidase |
| PRX | Peroxiredoxin |
| MAP2 | Microtubule-associated protein 2 |
| H2O2 | hydrogen peroxide |
| PD98059 | ERK inhibitor |
| LY-294002 | AKT inhibitor |
| H2DCFDA | 2′,7′-dichlorofluorescin diacetate |
| ΔΨm | mitochondrial membrane potential |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | fetal bovine serum |
| PBS | phosphate-buffered saline |
| TBS | Tris-buffered saline |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide |
References
- Lopes, F.M.; Schröder, R.; da Frota Júnior, M.L.C.; Zanotto-Filho, A.; Müller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between proliferative and neuron-like SH-SY5Y cells as an in vitro model for parkinson disease studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Schiwy, N.; Brazda, N.; Müller, H.W. Enhanced regenerative axon growth of multiple fibre populations in traumatic spinal cord injury following scar-suppressing treatment. Eur. J. Neurosci. 2009, 30, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y human neuroblastoma cell line. J. Vis. Exp. JoVE 2016, 53193. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The SH-SY5Y cell line in Parkinson’s disease research: A systematic review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.; Biryukov, M.; Trefois, C.; Antony, P.M.; Hussong, R.; Lin, J.; Heinäniemi, M.; Glusman, G.; Köglsberger, S.; Boyd, O.; et al. Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for parkinson’s disease. BMC Genom. 2014, 15, 1154. [Google Scholar] [CrossRef] [PubMed]
- Kovalevich, J.; Langford, D. Considerations for the use of SH-SY5Y neuroblastoma cells in neurobiology. In Neuronal Cell Culture; Springer Protocols Humana Press: Totowa, NJ, USA, 2013; pp. 9–21. [Google Scholar]
- Dexter, D.T.; Jenner, P. Parkinson disease: From pathology to molecular disease mechanisms. Free Radic. Biol. Med. 2013, 62, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Duester, G. Retinoic acid synthesis and signaling during early organogenesis. Cell 2008, 134, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhang, Y.; Liang, L.; Wei, M.; Hu, W.; Li, X.; Huang, Q. Effect of graphene oxide on undifferentiated and retinoic acid-differentiated SH-SY5Y cells line. Nanoscale 2012, 4, 3861–3866. [Google Scholar] [CrossRef] [PubMed]
- Pence, J.C.; Shorter, N.A. In vitro differentiation of human neuroblastoma cells caused by vasoactive intestinal peptide. Cancer Res. 1990, 50, 5177–5183. [Google Scholar] [PubMed]
- Dayem, A.A.; Kim, B.; Gurunathan, S.; Choi, H.Y.; Yang, G.; Saha, S.K.; Han, D.; Han, J.; Kim, K.; Kim, J.H. Biologically synthesized silver nanoparticles induce neuronal differentiation of SH-SY5Y cells via modulation of reactive oxygen species, phosphatases, and kinase signaling pathways. Biotechnol. J. 2014, 9, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, J.-H. Graphene oxide–silver nanoparticles nanocomposite stimulates differentiation in human neuroblastoma cancer cells (SH-SY5Y). Int. J. Mol. Sci. 2017, 18, 2549. [Google Scholar] [CrossRef] [PubMed]
- Cañón, E.; Cosgaya, J.M.; Scsucova, S.; Aranda, A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol. Biol. Cell 2004, 15, 5583–5592. [Google Scholar] [CrossRef] [PubMed]
- Konta, T.; Xu, Q.; Furusu, A.; Nakayama, K.; Kitamura, M. Selective roles of retinoic acid receptor and retinoid x receptor in the suppression of apoptosis by all-trans-retinoic acid. J. Biol. Chem. 2001, 276, 12697–12701. [Google Scholar] [CrossRef] [PubMed]
- Das, N.P. Effects of vitamin a and its analogs on nonenzymatic lipid peroxidation in rat brain mitochondria. J. Neurochem. 1989, 52, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Ahlemeyer, B.; Bauerbach, E.; Plath, M.; Steuber, M.; Heers, C.; Tegtmeier, F.; Krieglstein, J. Retinoic acid reduces apoptosis and oxidative stress by preservation of sod protein level. Free Radic. Biol. Med. 2001, 30, 1067–1077. [Google Scholar] [CrossRef]
- Ahlemeyer, B.; Krieglstein, J. Retinoic acid reduces staurosporine-induced apoptotic damage in chick embryonic neurons by suppressing reactive oxygen species production. Neurosci. Lett. 1998, 246, 93–96. [Google Scholar] [CrossRef]
- Nowack, B.; Krug, H.F.; Height, M. 120 years of nanosilver history: Implications for policy makers. Environ. Sci. Technol. 2011, 45, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Elechiguerra, J.L.; Burt, J.L.; Morones, J.R.; Camacho-Bragado, A.; Gao, X.; Lara, H.H.; Yacaman, M.J. Interaction of silver nanoparticles with HIV-1. J. Nanobiotechnol. 2005, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-J.; Sung, W.S.; Suh, B.K.; Moon, S.-K.; Choi, J.-S.; Kim, J.G.; Lee, D.G. Antifungal activity and mode of action of silver nano-particles on candida albicans. Biometals 2009, 22, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Boucher, W.; Stern, J.; Kotsinyan, V.; Kempuraj, D.; Papaliodis, D.; Cohen, M.; Theoharides, T. Intravesical nanocrystalline silver decreases experimental bladder inflammation. J. Urol. 2008, 179, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Bhol, K.C.; Schechter, P.J. Effects of nanocrystalline silver (NPI 32101) in a rat model of ulcerative colitis. Digest. Dis. Sci. 2007, 52, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, S.; Datta, H.K.; Chaudhuri, S.; Malik, D.; Bandyopadhyay, A. In-vitro anti-cancer activity of shape controlled silver nanoparticles (AGNPS) in various organ specific cell lines. J. Mol. Liq. 2017, 242, 757–766. [Google Scholar] [CrossRef]
- Kang, K.; Lim, D.-H.; Choi, I.-H.; Kang, T.; Lee, K.; Moon, E.-Y.; Yang, Y.; Lee, M.-S.; Lim, J.-S. Vascular tube formation and angiogenesis induced by polyvinylpyrrolidone-coated silver nanoparticles. Toxicol. Lett. 2011, 205, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wong, K.K.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K. Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Rim, K.-T.; Song, S.-W.; Kim, H.-Y. Oxidative DNA damage from nanoparticle exposure and its application to workers’ health: A literature review. Saf. Health Work 2013, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zhu, C.; An, Z.; Jiang, Y.; Zhao, Y.; Wang, J.; Liu, X.; Hui, B.; Zhang, X.; Wang, Y. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int. J. Nanomed. 2014, 9, 2469. [Google Scholar] [CrossRef] [PubMed]
- Sintubin, L.; Verstraete, W.; Boon, N. Biologically produced nanosilver: Current state and future perspectives. Biotechnol. Bioeng. 2012, 109, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Alon, N.; Miroshnikov, Y.; Perkas, N.; Nissan, I.; Gedanken, A.; Shefi, O. Substrates coated with silver nanoparticles as a neuronal regenerative material. Int. J. Nanomed. 2014, 9, 23. [Google Scholar]
- Bhore, N.; Wang, B.-J.; Chen, Y.-W.; Liao, Y.-F. Critical roles of dual-specificity phosphatases in neuronal proteostasis and neurological diseases. Int. J. Mol. Sci. 2017, 18, 1963. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Han, Y.-M.; Oh, M.; Kim, W.-K.; Oh, K.-J.; Lee, S.C.; Bae, K.-H.; Han, B.-S. DUSP4 regulates neuronal differentiation and calcium homeostasis by modulating ERK1/2 phosphorylation. Stem Cells Dev. 2014, 24, 686–700. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Sánchez-Olea, R.; Reyes-Reyes, E.M.; Panayiotidis, M.I. Environmental toxicity, oxidative stress and apoptosis: Menage a trois. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2009, 674, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Riggio, C.; Calatayud, M.P.; Hoskins, C.; Pinkernelle, J.; Sanz, B.; Torres, T.E.; Ibarra, M.R.; Wang, L.; Keilhoff, G.; Goya, G.F.; et al. Poly-l-lysine-coated magnetic nanoparticles as intracellular actuators for neural guidance. Int. J. Nanomed. 2012, 7, 3155. [Google Scholar]
- Riggio, C.; Calatayud, M.P.; Giannaccini, M.; Sanz, B.; Torres, T.E.; Fernández-Pacheco, R.; Ripoli, A.; Ibarra, M.R.; Dente, L.; Cuschieri, A.; et al. The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Richert, L.; Vetrone, F.; Yi, J.H.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Surface nanopatterning to control cell growth. Adv. Mater. 2008, 20, 1488–1492. [Google Scholar] [CrossRef]
- Kim, J.A.; Lee, N.; Kim, B.H.; Rhee, W.J.; Yoon, S.; Hyeon, T.; Park, T.H. Enhancement of neurite outgrowth in PC12 cells by iron oxide nanoparticles. Biomaterials 2011, 32, 2871–2877. [Google Scholar] [CrossRef] [PubMed]
- Paviolo, C.; Haycock, J.W.; Yong, J.; Yu, A.; Stoddart, P.R.; McArthur, S.L. Laser exposure of gold nanorods can increase neuronal cell outgrowth. Biotechnol. Bioeng. 2013, 110, 2277–2291. [Google Scholar] [CrossRef] [PubMed]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Hartemann, P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver nanoparticles (AGNPS) cause degeneration of cytoskeleton and disrupt synaptic machinery of cultured cortical neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.; Evje, L.; Syversen, T. Silver nanoparticle-induced cytotoxicity in rat brain endothelial cell culture. Toxicol. In Vitro 2013, 27, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Shen, W.; Gurunathan, S. Silver nanoparticle-mediated cellular responses in various cell lines: An in vitro model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Butler, K.S.; Peeler, D.J.; Casey, B.J.; Dair, B.J.; Elespuru, R.K. Silver nanoparticles: Correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis 2015, 30, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Rajeshkumar, S.; Kumar, V. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour.-Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Kikuchi, F.; Kato, Y.; Furihata, K.; Kogure, T.; Imura, Y.; Yoshimura, E.; Suzuki, M. Formation of gold nanoparticles by glycolipids of lactobacillus casei. Sci. Rep. 2016, 6, 34626. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Zeinali, S.; Yazdani, M. Synthesis of biologically stable gold nanoparticles using imidazolium-based amino acid ionic liquids. Amino Acids 2012, 43, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Soltani Nejad, M.; Shahidi Bonjar, G.H.; Khaleghi, N. Biosynthesis of gold nanoparticles using streptomyces fulvissimus isolate. Nanomed. J. 2015, 2, 153–159. [Google Scholar]
- Nissan, I.; Schori, H.; Lipovsky, A.; Alon, N.; Gedanken, A.; Shefi, O. Effect of different densities of silver nanoparticles on neuronal growth. J. Nanopart. Res. 2016, 18, 221. [Google Scholar] [CrossRef]
- He, W.; Kienzle, A.; Liu, X.; Müller, W.E.; Elkhooly, T.A.; Feng, Q. In vitro effect of 30 nm silver nanoparticles on adipogenic differentiation of human mesenchymal stem cells. J. Biomed. Nanotechnol. 2016, 12, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, W.; Fang, Z.; Kienzle, A.; Feng, Q. Influence of silver nanoparticles on osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Nanotechnol. 2014, 10, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Samberg, M.E.; Loboa, E.G.; Oldenburg, S.J.; Monteiro-Riviere, N.A. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine 2012, 7, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Q.; Xu, L.X. Effects of silver nanoparticles on the in vitro culture and differentiation of human bone marrow-derived mesenchymal cells. In Materials Science Forum; Trans Tech Publications: Zurich, Switzerland, 2016; pp. 1307–1312. [Google Scholar]
- He, W.; Elkhooly, T.A.; Liu, X.; Cavallaro, A.; Taheri, S.; Vasilev, K.; Feng, Q. Silver nanoparticle based coatings enhance adipogenesis compared to osteogenesis in human mesenchymal stem cells through oxidative stress. J. Mater. Chem. B 2016, 4, 1466–1479. [Google Scholar] [CrossRef]
- Rajanahalli, P.; Stucke, C.J.; Hong, Y. The effects of silver nanoparticles on mouse embryonic stem cell self-renewal and proliferation. Toxicol. Rep. 2015, 2, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mahmood, M.; Xu, Y.; Watanabe, F.; Biris, A.S.; Hansen, D.K.; Inselman, A.; Casciano, D.; Patterson, T.A.; Paule, M.G.; et al. Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front. Neurosci. 2015, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, X.; Wei, Y.; Liu, W.; Li, S.; Yu, G.; Fu, X.; Cao, T.; Deng, X. Cytotoxicity of silver nanoparticles in human embryonic stem cell-derived fibroblasts and an L-929 cell line. J. Nanomater. 2012, 2012, 160145. [Google Scholar] [CrossRef] [Green Version]
- Han, J.W.; Gurunathan, S.; Choi, Y.-J.; Kim, J.-H. Dual functions of silver nanoparticles in F9 teratocarcinoma stem cells, a suitable model for evaluating cytotoxicity-and differentiation-mediated cancer therapy. Int. J. Nanomed. 2017, 12, 7529. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Gurunathan, S.; Jeong, J.-K.; Choi, Y.-J.; Kwon, D.-N.; Park, J.-K.; Kim, J.-H. Oxidative stress mediated cytotoxicity of biologically synthesized silver nanoparticles in human lung epithelial adenocarcinoma cell line. Nanoscale Res. Lett. 2014, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B Biointerfaces 2009, 73, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Sriram, M.I.; Kanth, S.B.M.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in dalton’s lymphoma ascites tumor model. Int. J. Nanomed. 2010, 5, 753. [Google Scholar]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.-H. Comparative assessment of the apoptotic potential of silver nanoparticles synthesized by bacillus tequilensis and calocybe indica in MDA-MB-231 human breast cancer cells: Targeting p53 for anticancer therapy. Int. J. Nanomed. 2015, 10, 4203. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Jeyaraj, M.; Kim, J.-H. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. BioMed Res. Int. 2013, 2013, 535796. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.; Keyse, S. Differential regulation of map kinase signalling by dual-specificity protein phosphatases. Oncogene 2007, 26, 3203–3213. [Google Scholar] [CrossRef] [PubMed]
- Comfort, K.K.; Maurer, E.I.; Braydich-Stolle, L.K.; Hussain, S.M. Interference of silver, gold, and iron oxide nanoparticles on epidermal growth factor signal transduction in epithelial cells. ACS Nano 2011, 5, 10000–10008. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Cazzaniga, A.; Perrotta, C.; Maier, J.A. Silver nanoparticles-induced cytotoxicity requires ERK activation in human bladder carcinoma cells. Toxicol. Lett. 2015, 237, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Rinna, A.; Magdolenova, Z.; Hudecova, A.; Kruszewski, M.; Refsnes, M.; Dusinska, M. Effect of silver nanoparticles on mitogen-activated protein kinases activation: Role of reactive oxygen species and implication in DNA damage. Mutagenesis 2014, 30, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Paul, P.; Lee, S.; Qiao, L.; Josifi, E.; Tiao, J.R.; Chung, D.H. Pi3k/Akt and ERK regulate retinoic acid-induced neuroblastoma cellular differentiation. Biochem. Biophys. Res. Commun. 2012, 424, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Miloso, M.; Villa, D.; Crimi, M.; Galbiati, S.; Donzelli, E.; Nicolini, G.; Tredici, G. Retinoic acid-induced neuritogenesis of human neuroblastoma SH-SY5Y cells is erk independent and Pkc dependent. J. Neurosci. Res. 2004, 75, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Choi, Y.G.; Baik, J.Y.; Han, S.Y.; Jeong, D.-w.; Bae, Y.S.; Kim, N.; Lee, S.Y. A crucial role for reactive oxygen species in rankl-induced osteoclast differentiation. Blood 2005, 106, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Tsatmali, M.; Walcott, E.C.; Makarenkova, H.; Crossin, K.L. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol. Cell. Neurosci. 2006, 33, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a Pi3k/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Coso, S.; Harrison, I.; Harrison, C.B.; Vinh, A.; Sobey, C.G.; Drummond, G.R.; Williams, E.D.; Selemidis, S. Nadph oxidases as regulators of tumor angiogenesis: Current and emerging concepts. Antioxid. Redox Signal. 2012, 16, 1229–1247. [Google Scholar] [CrossRef] [PubMed]
- Storz, P. Forkhead homeobox type o transcription factors in the responses to oxidative stress. Antioxid. Redox Signal. 2011, 14, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Kiningham, K.K.; Cardozo, Z.-A.; Cook, C.; Cole, M.P.; Stewart, J.C.; Tassone, M.; Coleman, M.C.; Spitz, D.R. All-trans-retinoic acid induces manganese superoxide dismutase in human neuroblastoma through NF-κb. Free Radic. Biol. Med. 2008, 44, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Silvis, A.M.; McCormick, M.L.; Spitz, D.R.; Kiningham, K.K. Redox balance influences differentiation status of neuroblastoma in the presence of all-trans retinoic acid. Redox Biol. 2016, 7, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Hsin, Y.-H.; Chen, C.-F.; Huang, S.; Shih, T.-S.; Lai, P.-S.; Chueh, P.J. The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol. Lett. 2008, 179, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Chairuangkitti, P.; Lawanprasert, S.; Roytrakul, S.; Aueviriyavit, S.; Phummiratch, D.; Kulthong, K.; Chanvorachote, P.; Maniratanachote, R. Silver nanoparticles induce toxicity in a549 cells via ros-dependent and ros-independent pathways. Toxicol. In Vitro 2013, 27, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Goldsmit, Y.; Erlich, S.; Pinkas-Kramarski, R. Neuregulin induces sustained reactive oxygen species generation to mediate neuronal differentiation. Cell. Mol. Neurobiol. 2001, 21, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Katoh, S.; Mitsui, Y.; KITANI, K.; SUZUKI, T. Hyperoxia induces the neuronal differentiated phenotype of PC12 cells via a sustained activity of mitogen-activated protein kinase induced by Bcl-2. Biochem. J. 1999, 338, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Yao, Y.; Li, J.; Zhang, X.; Li, C.; Cheng, Y.; Ding, G.; Liu, L.; Ding, Z. Essential role of ERK activation in neurite outgrowth induced by α-lipoic acid. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2011, 1813, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Ohtani-Kaneko, R.; Ono, K.; Okado, N.; Shiga, T. Developmental regulation of activated erk expression in the spinal cord and dorsal root ganglion of the chick embryo. Neurosci. Res. 2005, 52, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, purification and characterization of silver nanoparticles using escherichia coli. Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Pool, M.; Thiemann, J.; Bar-Or, A.; Fournier, A.E. Neuritetracer: A novel imageJ plugin for automated quantification of neurite outgrowth. J. Neurosci. Methods 2008, 168, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, R.J.; Polaniak, R.; Bułdak, Ł.; Żwirska-Korczala, K.; Skonieczna, M.; Monsiol, A.; Kukla, M.; Duława-Bułdak, A.; Birkner, E. Short-term exposure to 50 Hz ELF-EMF alters the cisplatin-induced oxidative response in AT478 murine squamous cell carcinoma cells. Bioelectromagnetics 2012, 33, 641–651. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′, 7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Perelman, A.; Wachtel, C.; Cohen, M.; Haupt, S.; Shapiro, H.; Tzur, A. JC-1: Alternative excitation wavelengths facilitate mitochondrial membrane potential cytometry. Cell Death Dis. 2012, 3, e430. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Nam, S.; Kim, J.; Das, R.; Choi, S.; Nguyen, T.; Quan, X.; Choi, S.; Chung, C.; Lee, E. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis. 2015, 6, e1976. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Sinha, M.; Chanda, D.; Roy, T.; Banerjee, K.; Munshi, S.; Patro, B.S.; Chakrabarti, S. Mitochondrial dysfunction mediated by quinone oxidation products of dopamine: Implications in dopamine cytotoxicity and pathogenesis of Parkinson’s disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2011, 1812, 663–673. [Google Scholar] [CrossRef] [PubMed]






| Genes | Forward Primer | Reverse Primer | |
|---|---|---|---|
| Neuronal markers | MAP2 | GCTCTCTGAAGAACATCCGC | GGGCTTTAGCATGCTCTCTG |
| β-tubulin III | TCTCACAAGTACGTGCCTCG | CTCCGTGTAGTGACCCTTGG | |
| DUSPs-related genes | Dusp-1 | ACCACAAGGCAGACATCAG | AAGGTAAGCAAGGCAGATGG |
| Dusp-2 | CAGCTGCTGCAGTTTGAGAC | AGCTGATTTCTGCCAGAGGA | |
| Dusp-3 | GATCTCAACGACCTGCTCTC | ATGGGTGATGCCTAGTTTCTG | |
| Dusp-4 | ACGGCTCTGTTGAATGTCTC | CAGTCCTTCACGGCATCG | |
| Dusp-6 | ACAAGCAAATCCCCATCTCG | CAGCCAAGCAATGTACCAAG | |
| Dusp-7 | AACCTACCCAACGCCTTC | CACCAGGACACCACACTTC | |
| Dusp-9 | GAGGCTTCAGCAGATTCCAG | ATTGAGGATGTAGCGGATGC | |
| Oxidative-related genes | SOD1 | GGTGGGCCAAAGGATGAAGAG | CCACAAGCCAAACGACTTCC |
| SOD2 | GCTCCGGTTTTGGGGTATCTG | GCGTTGATGTGAGGTTCCAG | |
| SOD3 | ATGCGTGCGCTACTGTGTTC | CTCCGCCGAGTCAGAGTTG | |
| CAT | CTCCGCCGAGTCAGAGTTG | CCTTTGCCTTGGAGTATTTGGTA | |
| GPX1 | CAGTCGGTGTATGCCTTCTCG | GAGGGACGCCACATTCTCG | |
| GPX2 | GGTAGATTTCAATACGTTCCGGG | TGACAGTTCTCCTGATGTCCAAA | |
| GPX3 | AGAGCCGGGGACAAGAGAA | ATTTGCCAGCATACTGCTTGA | |
| GPX4 | GAGGCAAGACCGAAGTAAACTAC | CCGAACTGGTTACACGGGAA | |
| Housekeeping gene | GAPDH | AATCCCATCACCATCTTCCAG | AAATGAGCCCCAGCCTTC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdal Dayem, A.; Lee, S.B.; Choi, H.Y.; Cho, S.-G. Silver Nanoparticles: Two-Faced Neuronal Differentiation-Inducing Material in Neuroblastoma (SH-SY5Y) Cells. Int. J. Mol. Sci. 2018, 19, 1470. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051470
Abdal Dayem A, Lee SB, Choi HY, Cho S-G. Silver Nanoparticles: Two-Faced Neuronal Differentiation-Inducing Material in Neuroblastoma (SH-SY5Y) Cells. International Journal of Molecular Sciences. 2018; 19(5):1470. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051470
Chicago/Turabian StyleAbdal Dayem, Ahmed, Soo Bin Lee, Hye Yeon Choi, and Ssang-Goo Cho. 2018. "Silver Nanoparticles: Two-Faced Neuronal Differentiation-Inducing Material in Neuroblastoma (SH-SY5Y) Cells" International Journal of Molecular Sciences 19, no. 5: 1470. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051470