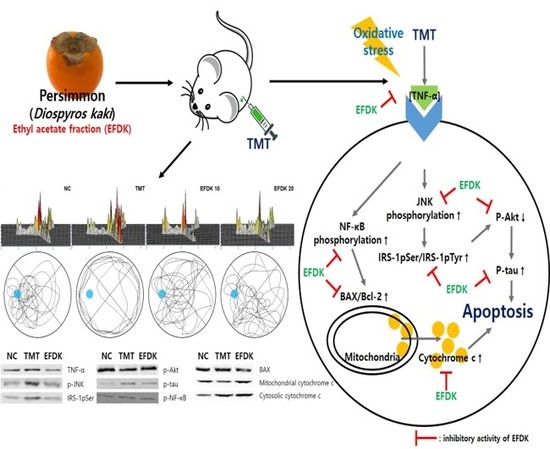
Ethyl Acetate Fraction from Persimmon (Diospyros kaki) Ameliorates Cerebral Neuronal Loss and Cognitive Deficit via the JNK/Akt Pathway in TMT-Induced Mice
Abstract
:1. Introduction
2. Results
2.1. Antioxidant Capacity
2.2. Protective Effect on H2O2-Induced Neurotoxicity in Hippocampal HT22 Cells
2.3. Y-Maze Test and Passive Avoidance Test
2.4. Passive Avoidance Test
2.5. Morris Water Maze Test
2.6. Ferric-Reducing Ability of Plasma (FRAP) and Antioxidant System in Brain Tissue
2.7. Cholinergic System in Brain Tissue
2.8. Cerebral Mitochondrial Function
2.9. Neuronal JNK/Akt Pathway
2.10. Neuronal Apoptotic Pathway
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Total Phenolic and Flavonoid Contents
4.3. Evaluation of Antioxidant Activity
4.4. Lipid Peroxide
4.5. Cell Culture and Treatment
4.6. Viability and Intracellular Reactive Oxygen Species (ROS)
4.7. Animals
4.8. Behavioral Tests
4.9. Plasma FRAP
4.10. Brain Tissue Preparation
4.11. Lipid Peroxidation
4.12. Superoxide Dismutase (SOD) Level
4.13. Reduced Gluthathione (GSH) Contents
4.14. Evaluation of Cholinergic System
4.15. Cerebral Mitochondrial Extraction
4.16. Estimation of Mitochondrial Function
4.17. Western Blot Analysis
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| ROS | reactive oxygen species |
| NFT | neurofibrillary tangles |
| TMT | trimethyltin |
| EFDK | ethyl acetate fraction from persimmon |
| TPC | total phenolic contents |
| TFC | total flavonoid contents |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH | reduced glutathione |
| AChE | acetylcholinesterase |
| ACh | acetylcholine |
| MMP | mitochondrial membrane potential |
| JNK | c-Jun N-terminal kinase |
| Akt | protein kinase B |
| TNF-α | tumor necrosis factor-alpha |
| IRS-1pSer | phosphorylated insulin receptor substrate 1 |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| BAX | Bcl-2-associated X protein |
| Aβ | amyloid-beta |
References
- Sokoloff, L. Energetics of functional activation in neural tissues. Neurochem. Res. 1999, 24, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Mullan, M.; Crawford, F. The molecular genetics of Alzheimer’s disease. Mol. Neurobiol. 1994, 9, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of oxidative stress in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.V.; Stoothoff, W.H. Tau phosphorylation in neuronal cell function and dysfunction. J. Cell. Sci. 2004, 117, 5721–5729. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Sharma, S.K. Plants as natural antioxidants. Nat. Prod. Radiance 2006, 5, 326–334. [Google Scholar]
- Butt, M.S.; Sultan, M.T.; Aziz, M.; Naz, A.; Ahmed, W.; Kumar, N.; Imran, M. Persimmon (Diospyros Kaki) fruit: Hidden phytochemicals and health claims. EXCLI J. 2015, 14, 542–561. [Google Scholar] [PubMed]
- Chen, J.; Du, J.; Ge, Z.; Zhu, W.; Nie, R.; Li, C. Comparison of sensory and compositions of five selected persimmon cultivars (Diospyros Kaki L.) and correlations between chemical components and processing characteristics. J. Food Sci. Technol. 2016, 53, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Cvikrová, M.; Martincová, O.; Ham, K.; Kang, S.; Park, Y.; Namiesnik, J.; Rombolà, A.D.; Jastrzebski, Z.; Gorinstein, S. In vitro antioxidative and binding properties of phenolics in traditional, citrus and exotic fruits. Food Res. Int. 2015, 74, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kubo, I.; Xiao, P.; Fujita, K. Antifungal activity of octyl gallate: Structural criteria and mode of action. Bioorg. Med. Chem. Lett. 2001, 11, 347–350. [Google Scholar] [CrossRef]
- Gato, N.; Kadowaki, A.; Hashimoto, N.; Yokoyama, S.; Matsumoto, K. Persimmon fruit tannin-rich fiber reduces cholesterol levels in humans. Ann. Nutr. MeTab. 2013, 62, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W.; Dyer, R.S. A time-course study of trimethyltin induced neuropathology in rats. Neurobehav. Toxicol. Teratol. 1983, 5, 443–459. [Google Scholar] [PubMed]
- Cannon, R.L.; Hoover, D.B.; Baisden, R.H.; Woodruff, M.L. Effects of trimethyltin (TMT) on choline acetyltransferase activity in the rat hippocampus. Mol. Chem. Neuropathol. 1994, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Brabeck, C.; Michetti, F.; Geloso, M.C.; Corvino, V.; Goezalan, F.; Meyermann, R.; Schluesener, H.J. Expression of EMAP-II by activated monocytes/microglial cells in different regions of the rat hippocampus after trimethyltin-induced brain damage. Exp. Neurol. 2002, 177, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Nishiyama, N.; Shuto, M.; Sugiyama, C.; Kawada, K.; Seko, K.; Nagashima, R.; Ogita, K. In vivo depletion of endogenous glutathione facilitates trimethyltin-induced neuronal damage in the dentate gyrus of mice by enhancing oxidative stress. Neurochem. Int. 2008, 52, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Schwarzer, C.; Furtinger, S.; Imai, H.; Kato, N.; Sperk, G. Changes in the GABA-ergic system induced by trimethyltin application in the rat. Mol. Brain Res. 2001, 97, 1–6. [Google Scholar] [CrossRef]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Chen, X.; Fan, J.; Yue, X.; Wu, X.; Li, L. Radical scavenging activity and phenolic compounds in persimmon (Diospyros Kaki L. cv. Mopan). J. Food Sci. 2008, 73, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Xie, Z.; Xu, X.; Yang, D. Persimmon (Diospyros Kaki L.) leaves: A review on traditional uses, phytochemistry and pharmacological properties. J. Ethnopharmacol. 2015, 163, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Sagara, Y.; Liu, Y.; Maher, P.; Schubert, D. Peroxide production in oxidative glutamate toxicity. J. Cell Biol. 1998, 141, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Murphy, T.H.; Miyamoto, M.; Sastre, A.; Schnaar, R.L.; Coyle, J.T. Glutamate toxicity in a neuronal cell line involves inhibition of cystine transport leading to oxidative stress. Neuron 1989, 2, 1547–1558. [Google Scholar] [CrossRef]
- Dellu, F.; Mayo, W.; Cherkaoui, J.; Le Moal, M.; Simon, H. A Two-trial memory task with automated recording: Study in young and aged rats. Brain Res. 1992, 588, 132–139. [Google Scholar] [CrossRef]
- Glenner, G.G. Alzheimer’s disease. The commonest form of amyloidosis. Arch. Pathol. Lab. Med. 1983, 107, 281–282. [Google Scholar] [PubMed]
- Huang, S.; Wang, W.; Zhang, M.; Liu, Q.; Luo, S.; Peng, Y.; Sun, B.; Wu, D.; Song, S. The effect of ethyl acetate extract from persimmon leaves on Alzheimer’s disease and its underlying mechanism. Phytomedicine 2016, 23, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zou, B.; Yang, L.; Xu, S.; Yang, J.; Yao, P.; Li, C. High molecular weight persimmon tannin ameliorates cognition deficits and attenuates oxidative damage in senescent mice induced by d-galactose. Food Chem. Toxicol. 2011, 49, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Naghizadeh, B.; Ghorbanzadeh, B.; Farbood, Y.; Sarkaki, A.; Bavarsad, K. Gallic acid prevents memory deficits and oxidative stress induced by intracerebroventricular injection of streptozotocin in rats. Pharmacol. Biochem. Behav. 2013, 111, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A.; Carney, J.M. Free radical damage to protein and DNA: Mechanisms involved and relevant observations on brain undergoing oxidative stress. Ann. Neurol. 1992, 32, 22–27. [Google Scholar] [CrossRef]
- Praticò, D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy. Ann. N. Y. Acad. Sci. 2008, 1147, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Gorinstein, S.; Kulasek, G.W.; Bartnikowska, E.; Leontowicz, M.; Zemser, M.; Morawiec, M.; Trakhtenberg, S. The influence of persimmon peel and persimmon pulp on the lipid metabolism and antioxidant activity of rats fed cholesterol. J. Nutr. Biochem. 1998, 9, 223–227. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Geerts, H. Indicators of neuroprotection with galantamine. Brain Res. Bull. 2005, 64, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; LaFerla, F.M. The role of nicotinic acetylcholine receptors in Alzheimer’s disease. J. Physiol.-Paris 2006, 99, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Kawai, H.; Berg, D.K. Beta-amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc. Natl. Acad. Sci. USA 2001, 98, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, K. Towards a unifying hypothesis of Alzheimer’s disease: Cholinergic system linked to plaques, tangles and neuroinflammation. Curr. Med. Chem. 2006, 13, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.E.; Khan, M.M.; Javed, H.; Vaibhav, K.; Khan, A.; Tabassum, R.; Ashafaq, M.; Islam, F.; Safhi, M.M.; Islam, F. Amelioration of cognitive impairment and neurodegeneration by catechin hydrate in rat model of streptozotocin-induced experimental dementia of Alzheimer’s type. Neurochem. Int. 2013, 62, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Lee, H.; Kim, J.; Hong, S.; Kim, H.; Jo, T.; Park, Y.; Lee, C.; Kim, Y.; Lee, S. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Whetsell, W.O., Jr. Current concepts of excitotoxicity. J. Neuropathol. Exp. Neurol. 1996, 55, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Rosenberg, P.A. Excitatory amino acids as a final common pathway for neurologic disorders. N. Engl. J. Med. 1994, 330, 613–622. [Google Scholar] [PubMed]
- Wong-Riley, M.T. Cytochrome oxidase: An endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989, 12, 94–101. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB signaling in inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Buchkovich, N.J.; Yu, Y.; Zampieri, C.A.; Alwine, J.C. The TORrid affairs of viruses: Effects of mammalian DNA viruses on the PI3K–Akt–mTOR signalling pathway. Nat. Rev. Microbiol. 2008, 6, 266. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Planel, E. Regulation of brain insulin signaling: A new function for tau. J. Exp. Med. 2017, 214, 2171–2173. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Atlas, R.; Lange, A.; Ginzburg, I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3Kinase signalling mechanism. Eur. J. Neurosci. 2005, 22, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Y.; Shi, Z.; Lu, D.; Li, T.; Ding, Y.; Ruan, Y.; Xu, A. The neuroprotection of liraglutide against ischaemia-induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Sci. Rep. 2016, 6, 26859. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Kim, M.O. Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017, 7, 40753. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Fu, J.; Zhao, Z.; Kong, X.; Huang, H.; Luo, L.; Yin, Z. Chlorogenic acid inhibits lipopolysaccharide-induced cyclooxygenase-2 expression in RAW264. 7 cells through suppressing NF-κB and JNK/AP-1 activation. Int. Immunopharmacol. 2009, 9, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ma, B.; Shang, Y.; Yin, Q.; Hong, Y.; Xu, S.; Shen, C.; Hou, X.; Liu, X. Flavonoid-rich ethanol extract from the leaves of Diospyros Kaki attenuates cognitive deficits, amyloid-beta production, oxidative stress, and neuroinflammation in APP/PS1 transgenic mice. Brain Res. 2018, 1678, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Abeysinghe, D.; Li, X.; Sun, C.; Zhang, W.; Zhou, C.; Chen, K. Bioactive Compounds and antioxidant capacities in different edible tissues of citrus fruit of four species. Food Chem. 2007, 104, 1338–1344. [Google Scholar] [CrossRef]
- Chang, S.; Wu, J.; Wang, S.; Kang, P.; Yang, N.; Shyur, L. Antioxidant activity of extracts from acacia confusa bark and heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199. [Google Scholar] [CrossRef]
- Davis, J.B.; Maher, P. Protein kinase C activation inhibits glutamate-induced cytotoxicity in a neuronal cell line. Brain Res. 1994, 652, 169–173. [Google Scholar] [CrossRef]
- Heo, H.J.; Cho, H.; Hong, B.; Kim, H.; Kim, E.; Kim, B.; Shin, D. Protective effect of 4′,5-dihydroxy-3′,6,7-trimethoxyflavone from Artemisia Asiatica against Aβ-induced oxidative stress in PC12 cells. Amyloid 2001, 8, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Van der Borght, K.; Havekes, R.; Bos, T.; Eggen, B.J.; Van der Zee, E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: Relationship with hippocampal neurogenesis. Behav. Neurosci. 2007, 121, 324. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.P.; Kosson, D.S. Passive avoidance learning in psychopathic and nonpsychopathic offenders. J. Abnorm. Psychol. 1986, 95, 252. [Google Scholar] [CrossRef] [PubMed]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, F.; Ng, T. Effect of pineal indoles on activities of the antioxidant defense enzymes superoxide dismutase, catalase, and glutathione reductase, and levels of reduced and oxidized glutathione in rat tissues. Biochem. Cell Biol. 2000, 78, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A New and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Vincent, D.; Segonzac, G.; Vincent, M.C. Colorimetric determination of acetylcholine by the hestrin hydroxylamine reaction and its application in pharmacy. Ann. Pharm. Fr. 1958, 16, 179–185. [Google Scholar] [PubMed]
- Brown, M.R.; Geddes, J.W.; Sullivan, P.G. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J. Bioenerg. Biomembr. 2004, 36, 401–406. [Google Scholar] [CrossRef] [PubMed]











| TPC a | TFC b | ABTS c | DPPH d | MDA e |
|---|---|---|---|---|
| 81.75 ± 1.52 | 187.52 ± 3.21 | 25.03 | 35.47 | 389.21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Kim, C.-W.; Lee, U.; Kim, S.-H.; Heo, H.J. Ethyl Acetate Fraction from Persimmon (Diospyros kaki) Ameliorates Cerebral Neuronal Loss and Cognitive Deficit via the JNK/Akt Pathway in TMT-Induced Mice. Int. J. Mol. Sci. 2018, 19, 1499. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051499
Kim JM, Park SK, Kang JY, Park SB, Yoo SK, Han HJ, Kim C-W, Lee U, Kim S-H, Heo HJ. Ethyl Acetate Fraction from Persimmon (Diospyros kaki) Ameliorates Cerebral Neuronal Loss and Cognitive Deficit via the JNK/Akt Pathway in TMT-Induced Mice. International Journal of Molecular Sciences. 2018; 19(5):1499. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051499
Chicago/Turabian StyleKim, Jong Min, Seon Kyeong Park, Jin Yong Kang, Su Bin Park, Seul Ki Yoo, Hye Ju Han, Chul-Woo Kim, Uk Lee, Sea-Hyun Kim, and Ho Jin Heo. 2018. "Ethyl Acetate Fraction from Persimmon (Diospyros kaki) Ameliorates Cerebral Neuronal Loss and Cognitive Deficit via the JNK/Akt Pathway in TMT-Induced Mice" International Journal of Molecular Sciences 19, no. 5: 1499. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051499