Overview of Cadmium Thyroid Disrupting Effects and Mechanisms
Abstract
:1. Introduction
2. Cadmium Effects on Thyroid Function
2.1. Human Studies
2.2. Animal Studies
2.2.1. Studies in Lower Vertebrates
2.2.2. Studies in Higher Vertebrates (Mammals)
3. Mechanisms of Cadmium Thyrotoxicity
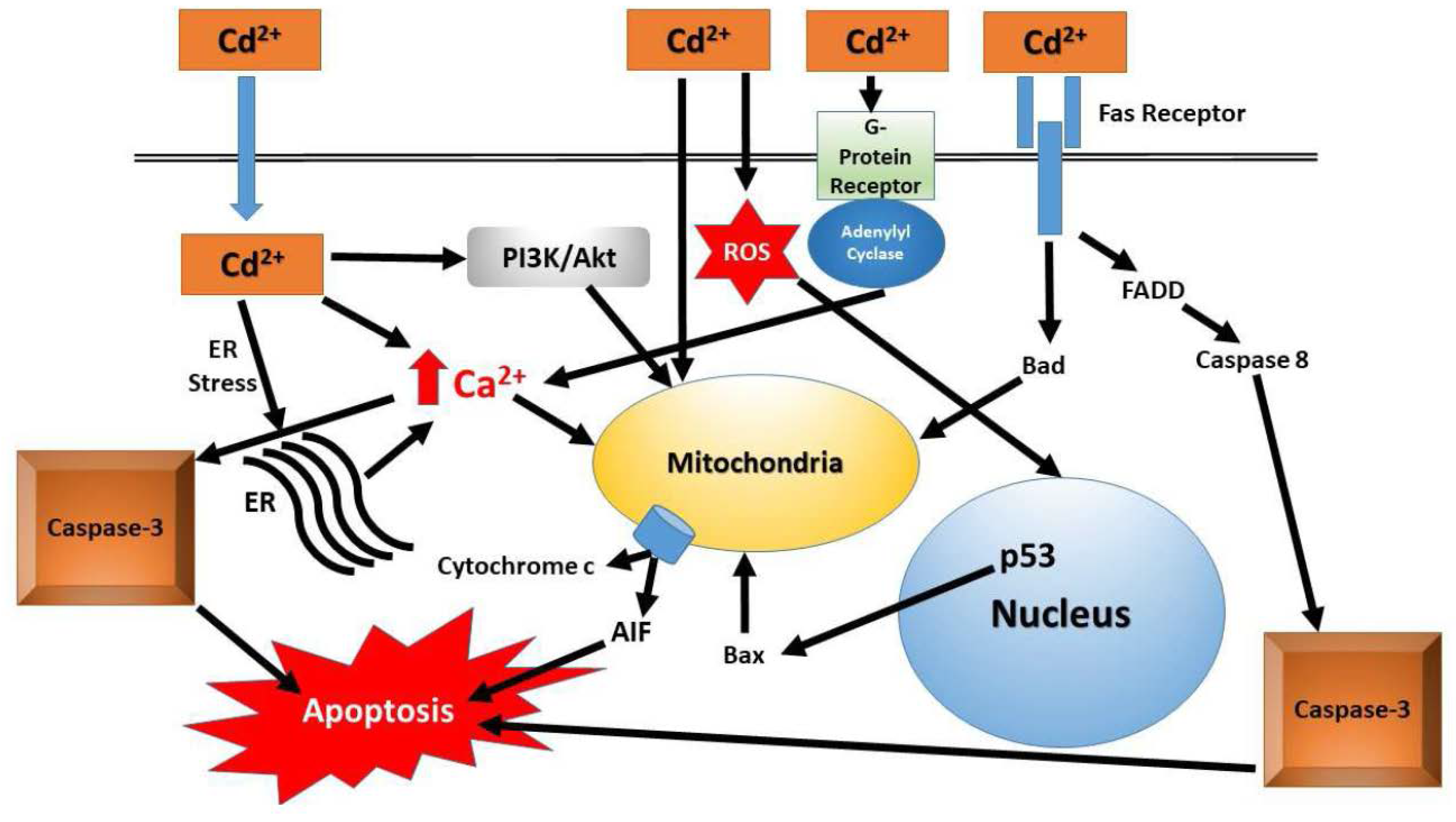
3.1. Primary Endocrine Toxicity
3.2. Secondary Endocrine Toxicity
4. Cadmium Nonmonotonic Effects Related to Thyroid Dysfunction
5. Cadmium and Thyroid Cancer
6. Conclusions and Future Research Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schug, T.T.; Johnson, A.F.; Birnbaum, L.S.; Colborn, T.; Guillette, L.J.; Crews, D.P.; Collins, T.; Soto, A.M.; vom Saal, F.S.; McLachlan, J.A.; et al. Minireview: Endocrine Disruptors: Past Lessons and Future Directions. Mol. Endocrinol. 2016, 30, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Petrakis, D.; Vassilopoulou, L.; Mamoulakis, C.; Psycharakis, C.; Anifantaki, A.; Sifakis, S.; Docea, A.O.; Tsiaoussis, J.; Makrigiannakis, A.; Tsatsakis, A.M. Endocrine disruptors leading to obesity and related diseases. Int. J. Environ. Res. Public Health 2017, 14, 1282. [Google Scholar] [CrossRef] [PubMed]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Pharmacol. 2017, 51, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, S. Thyroid Disorders During Pregnancy. Med. Clin. N. Am. 2012, 96, 235–256. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.R. Neurodevelopmental and neurophysiological actions of thyroid hormone. J. Neuroendocrinol. 2008, 20, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Danzi, S.; Klein, I. Thyroid Hormone and the Cardiovascular System. Med. Clin. N. Am. 2012, 96, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G. Peripheral metabolism of thyroid hormones: A review. Altern. Med. Rev. 2000, 5, 306–333. [Google Scholar] [PubMed]
- Harvey, C.B.; Williams, G.R. Mechanism of thyroid hormone action. Thyroid 2002, 12, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.K.; Salwan, P.; Salwan, S. Various possible toxicants involved in thyroid dysfunction: A review. J. Clin. Diagn. Res. 2016, 10, FE01–FE03. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chen, Y.; Chen, Y.; Chen, C.; Han, B.; Li, Q.; Zhu, C.; Xia, F.; Zhai, H.; Wang, N.; et al. Lead and cadmium exposure, higher thyroid antibodies and thyroid dysfunction in Chinese women. Environ. Pollut. 2017, 230, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.S.; Brix, T.H.; Iachine, I.; Kyvik, K.O.; Hegedüs, L. The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: A study of healthy Danish twins. Eur. J. Endocrinol. 2006, 154, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Pellegriti, G.; Frasca, F.; Regalbuto, C.; Squatrito, S.; Vigneri, R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013, 2013, 965212. [Google Scholar] [CrossRef] [PubMed]
- Vigneri, R.; Malandrino, P.; Gianì, F.; Russo, M.; Vigneri, P. Heavy metals in the volcanic environment and thyroid cancer. Mol. Cell. Endocrinol. 2017, 457, 73–80. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Cadmium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA; The Public Health Service: Washington, DC, USA; The U.S. Department of Health and Human Services: Washington, DC, USA, 2012; pp. 1–487. [Google Scholar]
- Cadmium—Water Quality Association. Available online: https://www.wqa.org/Portals/0/Technical/Technical%20Fact%20Sheets/2015_Cadmium.pdf (accessed on 13 March 2018).
- Klaassen, C.D.; Liu, J.; Diwan, B.A. Metallothionein protection of cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Uetani, M.; Kobayashi, E.; Suwazono, Y.; Honda, R.; Nishijo, M.; Nakagawa, H.; Kido, T.; Nogawa, K. Tissue cadmium (Cd) concentrations of people living in a Cd polluted area, Japan. BioMetals 2006, 19, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Matović, V.; Buha, A.; Bulat, Z.; Đukić-Ćosić, D. Cadmium Toxicity Revisited: Focus on Oxidative Stress Induction and Interactions with Zinc and Magnesium. Arch. Ind. Hyg. Toxicol. 2011, 62, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Matović, V.; Buha, A.; Dukić-Ćosić, D.; Bulat, Z. Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food Chem. Toxicol. 2015, 78, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Mezynska, M.; Brzóska, M.M. Environmental exposure to cadmium—A risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. 2018, 25, 3211–3232. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.; Ackerman, C. A Review of Diabetes Mellitus and Exposure to the Environmental Toxicant Cadmium with an Emphasis on Likely Mechanisms of Action. Curr. Diabetes Rev. 2016, 12, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Peiris-John, R.; Wickremasinghe, R.; Senanayake, H.; Sathiakumar, N. Cadmium a metalloestrogen: Are we convinced? J. Appl. Toxicol. 2012, 32, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.Z.; Schisterman, E.F.; Goldman, L.R.; Mumford, S.L.; Albert, P.S.; Jones, R.L.; Wactawski-Wende, J. Cadmium, lead, and mercury in relation to reproductive hormones and anovulation in premenopausal women. Environ. Health Perspect. 2011, 119, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- IARC. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans International Agency for Research on Cancer Iarc Monographs on The Evaluation of Carcinogenic Risks to Humans. In Personal Habits and Indoor Combustions; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100. [Google Scholar]
- Larsson, S.C.; Orsini, N.; Wolk, A. Urinary cadmium concentration and risk of breast cancer: A systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2015, 182, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Wallace, D.; Matovic, V.; Schweitzer, A.; Oluic, B.; Micic, D.; Djordjevic, V. Cadmium Exposure as a Putative Risk Factor for the Development of Pancreatic Cancer: Three Different Lines of Evidence. Biomed Res. Int. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Feki-Tounsi, M.; Hamza-Chaffai, A. Cadmium as a possible cause of bladder cancer: A review of accumulated evidence. Environ. Sci. Pollut. Res. 2014, 21, 10561–10573. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Kim, S.S.; Chung, E.; Dietrich, K.N. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the national health and nutrition examination survey, 2007–2008. Environ. Health Perspect. 2013, 121, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Yorita Christensen, K.L. Metals in blood and urine, and thyroid function among adults in the United States 2007–2008. Int. J. Hyg. Environ. Health 2013, 216, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B.; Choi, Y.S. Interacting effects of selected trace and toxic metals on thyroid function. Int. J. Environ. Health Res. 2016, 26, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hendryx, M. Relationship between blood cadmium, lead, and serum thyroid measures in US adults—The National Health and Nutrition Examination Survey (NHANES) 2007–2010. Int. J. Environ. Health Res. 2014, 24, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Rosati, M.V.; Montuori, L.; Caciari, T.; Sacco, C.; Marrocco, M.; Tomei, G.; Scala, B.; Sancini, A.; Anzelmo, V.; Bonomi, S.; et al. Correlation between urinary cadmium and thyroid hormones in outdoor workers exposed to urban stressors. Toxicol. Ind. Health 2016, 32, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Jurdziak, M.; Gać, P.; Poręba, M.; Szymańska-Chabowska, A.; Mazur, G.; Poręba, R. Concentration of Thyrotropic Hormone in Persons Occupationally Exposed to Lead, Cadmium and Arsenic. Biol. Trace Elem. Res. 2018, 182, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.M.; Mohammed, Y.S.; Zedan, H.A.E. Toxic Effect of Some Heavy Metals (Cadmium and Lead) on Thyroid Function. Egypt. J. Hosp. Med. 2017, 69, 2512–2515. [Google Scholar] [CrossRef]
- Meeker, J.D.; Rossano, M.G.; Protas, B.; Diamond, M.P.; Puscheck, E.; Daly, D.; Paneth, N.; Wirth, J.J. Multiple metals predict prolactin and thyrotropin (TSH) levels in men. Environ. Res. 2009, 109, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Rasic-Milutinovic, Z.; Jovanovic, D.; Bogdanovic, G.; Trifunovic, J.; Mutic, J. Potential Influence of Selenium, Copper, Zinc and Cadmium on l-Thyroxine Substitution in Patients with Hashimoto Thyroiditis and Hypothyroidism. Exp. Clin. Endocrinol. Diabetes 2017, 125, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, B.; Lin, K.; Zhang, Y.; Xu, X.; Huo, X. Thyroid disruption and reduced mental development in children from an informal E-waste recycling area: A mediation analysis. Chemosphere 2018, 193, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Otake, T.; Yoshinaga, J.; Ikegami, M.; Suzuki, E.; Naruse, H.; Yamanaka, T.; Shibuya, N.; Yasumizu, T.; Kato, N. Cadmium, lead, and selenium in cord blood and thyroid hormone status of newborns. Biol. Trace Elem. Res. 2007, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Nugegoda, D.; Kibria, G. Effects of environmental chemicals on fish thyroid function: Implications for fisheries and aquaculture in Australia. Gen. Comp. Endocrinol. 2017, 244, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Hontela, A.; Daniel, C.; Ricard, A.C. Effects of acute and subacute exposures to cadmium on the interrenal and thyroid function in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 1996, 35, 171–182. [Google Scholar] [CrossRef]
- Garcia-Santos, S.; Fontaínhas-Fernandes, A.; Monteiro, S.M.; Wilson, J.M. Effects of exposure to cadmium on some endocrine parameters in tilapia, Oreochromis niloticus. Bull. Environ. Contam. Toxicol. 2013, 90, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Jadhao, A.G.; Paul, P.L.; Rao, P.D. Effect of cadmium chloride on the pituitary, thyroid and gonads in the catfish, Clarias batrachus (Linn.). Funct. Dev. Morphol. 1994, 4, 39–44. [Google Scholar] [PubMed]
- Li, Z.H.; Chen, L.; Wu, Y.H.; Li, P.; Li, Y.F.; Ni, Z.H. Effects of waterborne cadmium on thyroid hormone levels and related gene expression in Chinese rare minnow larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 161, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Ricard, A.C.; Daniel, C.; Anderson, P.; Hontela, A. Effects of subchronic exposure to cadmium chloride on endocrine and metabolic functions in rainbow trout Oncorhynchus mykiss. Arch. Environ. Contam. Toxicol. 1998, 34, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, F.; Messaoudi, I.; El Hani, J.; Baati, T.; Saïd, K.; Kerkeni, A. Reversal of cadmium-induced thyroid dysfunction by selenium, zinc, or their combination in rat. Biol. Trace Elem. Res. 2008, 126, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.M.; Salama, A.F.; Nimr, T.M.E.; Gamal, D.M.E. Effects of phytate on thyroid gland of rats intoxicated with cadmium. Toxicol. Ind. Health 2015, 31, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, A.; Cano, P.; Esquifino, A.I. Are cadmium effects on plasma gonadotropins, prolactin, ACTH, GH and TSH levels, dose-dependent? BioMetals 2003, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Caride, A.; Fernández-Pérez, B.; Cabaleiro, T.; Tarasco, M.; Esquifino, A.I.; Lafuente, A. Cadmium chronotoxicity at pituitary level: Effects on plasma ACTH, GH, and TSH daily pattern. J. Physiol. Biochem. 2010, 66, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Zienab, Y.M.; Heba, H.E.-G. Effect of Cadmium Chloride on Function of Thyroid Gland in Rats. Egyptain J. Comp. Pathol. Clin. Pathol. 2009, 22, 10–23. [Google Scholar]
- Piłat-Marcinkiewicz, B.; Brzóska, M.M.; Sawicki, B.; Moniuszko-Jakoniuk, J. Structure and function of thyroid follicular cells in female rats chronically exposed to cadmium. Bull. Vet. Inst. Pulawy 2003, 47, 157–163. [Google Scholar]
- Buha, A.; Antonijević, B.; Bulat, Z.; Jaćević, V.; Milovanović, V.; Matović, V. The impact of prolonged cadmium exposure and co-exposure with polychlorinated biphenyls on thyroid function in rats. Toxicol. Lett. 2013, 221, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Crews, D.; Putz, O.; Thomas, P.; Hayes, T.; Howdeshell, K. Wildlife as models for the study of how mixtures, low doses, and the embryonic environment modulate the action of endocrine-disrupting chemicals. Pure Appl. Chem. 2003, 75, 2305–2320. [Google Scholar] [CrossRef]
- Yang, J.M.; Salmon, A.G.; Marty, M.A. Development of TEFs for PCB congeners by using an alternative biomarker—Thyroid hormone levels. Regul. Toxicol. Pharmacol. 2010, 56, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Klaassen, C.D. Differential effects of polychlorinated biphenyl congeners on serum thyroid hormone levels in rats. Toxicol. Sci. 2010, 117, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Antonijević, B.; Milovanović, V.; Janković, S.; Bulat, Z.; Matović, V. Polychlorinated biphenyls as oxidative stress inducers in liver of subacutely exposed rats: Implication for dose-dependence toxicity and benchmark dose concept. Environ. Res. 2015, 136, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ross, D.G.; Devito, M.J.; Crofton, K.M. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicol. Sci. 2001, 61, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Skarman, E.; Darnerud, P.O.; Öhrvik, H.; Oskarsson, A. Reduced thyroxine levels in mice perinatally exposed to polybrominated diphenyl ethers. Environ. Toxicol. Pharmacol. 2005, 19, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, V.; Buha, A.; Matovic, V.; Curcic, M.; Vucinic, S.; Nakano, T.; Antonijevic, B. Oxidative stress and renal toxicity after subacute exposure to decabrominated diphenyl ether in Wistar rats. Environ. Sci. Pollut. Res. 2018, 25, 7223–7230. [Google Scholar] [CrossRef] [PubMed]
- Ćurčić, M.; Janković, S.; Jaćević, V.; Stanković, S.; Vučinić, S.; Durgo, K.; Bulat, Z.; Antonijević, B. Combined effects of cadmium and decabrominated diphenyl ether on thyroid hormones in rats. Arh. Hig. Rada Toksikol. 2012, 63, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Wade, M.G.; Parent, S.; Finnson, K.W.; Foster, W.; Younglai, E.; McMahon, A.; Cyr, D.G.; Hughes, C. Thyroid toxicity due to subchronic exposure to a complex mixture of 16 organochlorines, lead, and cadmium. Toxicol. Sci. 2002, 67, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Yoshizuka, M.; Mori, N.; Hamasaki, K.; Tanaka, I.; Yokoyama, M.; Hara, K.; Doi, Y.; Umezu, Y.; Araki, H.; Sakamoto, Y. Cadmium toxicity in the thyroid gland of pregnant rats. Exp. Mol. Pathol. 1991, 55, 97–104. [Google Scholar] [CrossRef]
- Jancic, S.A.; Stosic, B.Z. Cadmium Effects on the Thyroid Gland. Vitam. Horm. 2014, 94, 391–425. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, H.; Ju, Z.; Zhao, H. Effects of chronic cadmium exposure on metamorphosis, skeletal development, and thyroid endocrine disruption in Chinese toad Bufo gargarizans tadpoles. Environ. Toxicol. Chem. 2018, 37, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Miyata, K.; Ose, K. Thyroid Hormone-disrupting Effects and the Amphibian Metamorphosis Assay. J. Toxicol. Pathol. 2012, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Prakash, P.; Kumar, P.G.; Laloraya, M.; Javeri, T.; Parihar, M.S. Superoxide anion radical production as a cadmium-mediated mechanism of toxicity in avian thyroid: An electron spin resonance study by spin trapping. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1997, 118, 89–95. [Google Scholar] [CrossRef]
- Alkharashi, N.A.O.; Periasamy, V.S.; Athinarayanan, J.; Alshatwi, A.A. Cadmium triggers mitochondrial oxidative stress in human peripheral blood lymphocytes and monocytes: Analysis using in vitro and system toxicology approaches. J. Trace Elem. Med. Biol. 2017, 42, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Al-Assaf, A.H.; Alqahtani, A.M.; Alshatwi, A.A.; Syed, N.A.; Shafi, G.; Hasan, T.N. Mechanism of cadmium induced apoptosis in human peripheral blood lymphocytes: The role of p53, Fas and Caspase-3. Environ. Toxicol. Pharmacol. 2013, 36, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Park, K.-K.; Lee, B.-H.; Moon, C.-K.; Lee, M.-O. Identification of genes that are induced after cadmium exposure by suppression subtractive hybridization. Toxicology 2003, 191, 121–131. [Google Scholar] [CrossRef]
- Hossain, S.; Liu, H.N.; Nguyen, M.; Shore, G.; Almazan, G. Cadmium exposure induces mitochondria-dependent apoptosis in oligodendrocytes. Neurotoxicology 2009, 30, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Korotkov, S.M.; Saris, N.E. In vitro modulation of heavy metal-induced rat liver mitochondria dysfunction: A comparison of copper and mercury with cadmium. J. Trace Elem. Med. Biol. 2011, 25, S63–S73. [Google Scholar] [CrossRef] [PubMed]
- Cannino, G.; Ferruggia, E.; Luparello, C.; Rinaldi, A.M. Cadmium and mitochondria. Mitochondrion 2009, 9, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Ikeda, K.; Sugiyama, H. Cytotoxicity of Heavy Metals to A Thyroid Carcinoma Cell Line. J. Environ. Anal. Toxicol. 2017, 7, 1–3. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kizu, R.; Sugiyama, H. Influences of Polyaromatic Hydrocarbons and Heavy. J. Health Sci. 2005, 51, 202–206. [Google Scholar] [CrossRef]
- Chen, G.G.; Liu, Z.M.; Vlantis, A.C.; Tse, G.M.K.; Leung, B.C.H.; van Hasselt, C.A. Heme oxygenase-1 protects against apoptosis induced by tumor necrosis factor-α and cycloheximide in papillary thyroid carcinoma cells. J. Cell. Biochem. 2004, 92, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, R.; Nagamine, T.; Takagi, H.; Mori, M.; Waalkes, M.P. Induction of apoptosis in cells by cadmium: Quantitative negative correlation between basal or induced metallothionein concentration and apoptotic rate. Toxicol. Sci. 2001, 64, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kar, A. Role of testosterone in ameliorating the cadmium induced inhibition of thyroid function in adult male mouse. Bull. Environ. Contam. Toxicol. 1997, 58, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Chaurasia, S.S.; Maiti, P.K.; Kar, A. Cadmium induced alterations in extrathyroidal conversion of thyroxine to triiodothyronine by type-I iodothyronine 5′-monodeiodinase in male mouse. Horm. Metab. Res. 1997, 29, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, S.S.; Gupta, P.; Kar, A.; Maiti, P.K. Free radical mediated membrane perturbation and inhibition of type-I iodothyronine 5′-monodeiodinase activity by lead and cadmium in rat liver homogenate. Biochem. Mol. Biol. Int. 1996, 39, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Buha, A.; Bulat, Z.; Dukić-Ćosić, D.; Matović, V. Effects of oral and intraperitoneal magnesium treatment against cadmiuminduced oxidative stress in plasma of rats. Arh. Hig. Rada Toksikol. 2012, 63, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Matović, V.; Buha, A.; Bulat, Z.; Dukić-ćosić, D.; Miljković, M.; Ivanišević, J.; Kotur-Stevuljević, J. Route-dependent effects of cadmium/cadmium and magnesium acute treatment on parameters of oxidative stress in rat liver. Food Chem. Toxicol. 2012, 50, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Al-Waeli, A.; Pappas, A.C.; Zoidis, E.; Georgiou, C.A.; Fegeros, K.; Zervas, G. The role of selenium in cadmium toxicity: Interactions with essential and toxic elements. Br. Poult. Sci. 2012, 53, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Priya, L.; Gupta, S. Effects of combined exposure to lead and cadmium on the hypothalamic-pituitary axis function in proestrous rats. Food Chem. Toxicol. 2003, 41, 379–384. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Zhang, K.; Huang, Y.; Yan, Y.; Wang, F.; Wu, J.; Wang, X.; Xu, Z.; Chen, Y.; et al. Cadmium-induced immune abnormality is a key pathogenic event in human and rat models of preeclampsia. Environ. Pollut. 2016, 218, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, Y.; Chai, L.; Wang, H. Oxidative stress, endocrine disruption, and malformation of Bufo gargarizans embryo exposed to sub-lethal cadmium concentrations. Environ. Toxicol. Pharmacol. 2017, 49, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Patiño, R. Exposure of xenopus laevis tadpoles to cadmium reveals concentration-dependent bimodal effects on growth and monotonic effects on development and thyroid gland activity. Toxicol. Sci. 2008, 105, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Vattai, A.; Ziegelmüller, B.; Kost, B.; Kuhn, C.; Hofmann, S.; Bayer, B.; Anslinger, K.; Jeschke, U.; Ditsch, N. The expression of thyroid hormone receptors (THR) is regulated by the progesterone receptor system in first trimester placental tissue and in BeWo cells in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Smida, A.D.; Valderrama, X.P.; Agostini, M.C.; Furlan, M.A.; Chedrese, J. Cadmium stimulates transcription of the cytochrome p450 side chain cleavage gene in genetically modified stable porcine granulosa cells. Biol. Reprod. 2004, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Renieri, E.A.; Sfakianakis, D.G.; Alegakis, A.A.; Safenkova, I.V.; Buha, A.; Matović, V.; Tzardi, M.; Dzantiev, B.B.; Divanach, P.; Kentouri, M.; et al. Nonlinear responses to waterborne cadmium exposure in zebrafish. An in vivo study. Environ. Res. 2017, 157, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, G.; Yu, Y.; Zhu, H. The hormetic effect of cadmium on the activity of antioxidant enzymes in the earthworm Eisenia fetida. Environ. Pollut. 2009, 157, 3064–3068. [Google Scholar] [CrossRef] [PubMed]
- Helmestam, M.; Stavreus-Evers, A.; Olovsson, M. Cadmium chloride alters mRNA levels of angiogenesis related genes in primary human endometrial endothelial cells grown in vitro. Reprod. Toxicol. 2010, 30, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Höfer, N.; Diel, P.; Wittsiepe, J.; Wilhelm, M.; Kluxen, F.M.; Degen, G.H. Investigations on the estrogenic activity of the metallohormone cadmium in the rat intestine. Arch. Toxicol. 2010, 84, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Duan, W.; Xu, L.; Song, S.; Zhu, C.; Wu, L. Biphasic effect of cadmium on cell proliferation in human embryo lung fibroblast cells and its molecular mechanism. Toxicol. In Vitro 2009, 23, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Falnoga, I.; Tušek-Žnidarič, M.; Horvat, M.; Stegnar, P. Mercury, selenium, and cadmium in human autopsy samples from Idrija residents and mercury mine workers. Environ. Res. 2000, 84, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.K.; Nam, J.S.; Ahn, C.W.; Lee, Y.S.; Kim, K.R. Some Elements in Thyroid Tissue are Associated with More Advanced Stage of Thyroid Cancer in Korean Women. Biol. Trace Elem. Res. 2016, 171, 135–720. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.M.; Fallahi, P.; Antonelli, A.; Benvenga, S. Environmental issues in thyroid diseases. Front. Endocrinol. (Lausanne) 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.V.S. Perspectives in endocrine toxicity of heavy metals—A review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Siewit, C.L.; Gengler, B.; Vegas, E.; Puckett, R.; Louie, M.C. Cadmium Promotes Breast Cancer Cell Proliferation by Potentiating the Interaction between ERα and c-Jun. Mol. Endocrinol. 2010, 24, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Lombaert, N.; Lison, D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ. Int. 2016, 86, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D. Nanotoxicology and Metalloestrogens: Possible Involvement in Breast Cancer. Toxics 2015, 3, 390–413. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Divekar, S.D.; Storchan, G.B.; Parodi, D.A.; Martin, M.B. Metals and Breast Cancer. J. Mammary Gland Biol. Neoplas. 2013, 18, 63–73. [Google Scholar] [CrossRef]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced cancers in animals and in humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, A. Cadmium and Cancer. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 491–507. ISBN 978-94-007-5179-8. [Google Scholar]
- Wallace, D.R. Expanding the Role of Cadmium in Pancreatic Cancer. EC Pharmacol. Toxicol. 2017, ECO.01, 19–21. [Google Scholar]
- Venza, M.; Visalli, M.; Biondo, C.; Oteri, R.; Agliano, F.; Morabito, S.; Caruso, G.; Caffo, M.; Teti, D.; Venza, I. Epigenetic Effects of Cadmium in Cancer: Focus on Melanoma. Curr. Genom. 2014, 15, 420–435. [Google Scholar] [CrossRef] [PubMed]
- Bishak, Y.K.; Payahoo, L.; Osatdrahimi, A.; Nourazarian, A. Mechanisms of cadmium carcinogenicity in the gastrointestinal tract. Asian Pac. J. Cancer Prev. 2015, 16, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Chen, G.G.; Vlantis, A.C.; Tse, G.M.; Shum, C.K.Y.; Van Hasselt, C.A. Calcium-mediated activation of PI3K and p53 leads to apoptosis in thyroid carcinoma cells. Cell. Mol. Life Sci. 2007, 64, 1428–1436. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Sugihira, N.; Suzuki, M.; Sakurada, T.; Saito, S.; Yoshinaga, K.; Saito, H. Effect of cadmium on T4 outer ring monodeiodination by rat liver. Environ. Res. 1987, 42, 400–405. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Kong, D.; Ren, S.; Li, N. T-screen and yeast assay for the detection of the thyroid-disrupting activities of cadmium, mercury, and zinc. Environ. Sci. Pollut. Res. 2016, 23, 9843–9851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.M.; Chen, G.G.; Shum, C.K.Y.; Vlantis, A.C.; George Cherian, M.; Koropatnick, J.; Andrew van Hasselt, C. Induction of functional MT1 and MT2 isoforms by calcium in anaplastic thyroid carcinoma cells. FEBS Lett. 2007, 581, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, R.; Zhang, L.; Kebebew, E. Thyroid cancer gender disparity. Future Oncol. 2010, 6, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Vannucchi, G.; De Leo, S.; Perrino, M.; Rossi, S.; Tosi, D.; Cirello, V.; Colombo, C.; Bulfamante, G.; Vicentini, L.; Fugazzola, L. Impact of estrogen and progesterone receptor expression on the clinical and molecular features of papillary thyroid cancer. Eur. J. Endocrinol. 2015, 173, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vivacqua, A.; Bonofiglio, D.; Albanito, L.; Madeo, A.; Rago, V.; Carpino, A.; Musti, A.M.; Picard, D.; Andò, S.; Maggiolini, M. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol. Pharmacol. 2006, 70, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Jalali-Nadoushan, M.-R.; Amirtouri, R. Expression of estrogen and progesterone receptors in subcutaneous endometriosis. Casp. J. Intern. Med. 2016, 7, 183–187. [Google Scholar] [CrossRef]
- Ronchetti, S.A.; Miler, E.A.; Duvilanski, B.H.; Cabilla, J.P. Cadmium Mimics Estrogen-Driven Cell Proliferation and Prolactin Secretion from Anterior Pituitary Cells. PLoS ONE 2013, 8, e81101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, P.; Liao, L.-Y.; Zhao, T.-T.; Mo, X.-M.; Chen, G.G.; Liu, Z.-M. GPER/ERK/AKT/NF-κB pathway is involved in cadmium-induced proliferation, invasion and migration of GPER-positive thyroid cancer cells. Mol. Cell. Endocrinol. 2017, 442, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gu, J.; Zhang, Y.; Wei, W.; Qu, N.; Wen, D.; Liao, T.; Shi, R.; Zhang, L.; Ji, Q.; et al. Aberrant hypermethylation of the HOXD10 gene in papillary thyroid cancer with BRAFV600E mutation. Oncol. Rep. 2018, 39, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Bisarro Dos Reis, M.; Barros-Filho, M.C.; Marchi, F.A.; Beltrami, C.M.; Kuasne, H.; Pinto, C.A.L.; Ambatipudi, S.; Herceg, Z.; Kowalski, L.P.; Rogatto, S.R. Prognostic Classifier Based on Genome-Wide DNA Methylation Profiling in Well-Differentiated Thyroid Tumors. J. Clin. Endocrinol. Metab. 2017, 102, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; De la Chapelle, A.; Pellegata, N.S. Hypermethylation, but not LOH, is associated with the low expression of MT1G and CRABP1 in papillary thyroid carcinoma. Int. J. Cancer 2003, 104, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G. Toxicological aspects of metallothionein. Cell. Mol. Biol. 2000, 46, 451–463. [Google Scholar] [PubMed]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G.F. Trace element research-historical and future aspects. J. Trace Elem. Med. Biol. 2016, 38, 46–52. [Google Scholar] [CrossRef] [PubMed]
| Cd Concentrations or Dose(s) | Exposure Duration | Species | Effects | Method of Quantifying | Reference |
|---|---|---|---|---|---|
| 0.4, 0.8 and 2.4 mg Cd/L | 2, 4, 24 or 96 h | juvenile rainbow trout (Oncorhynchus mykiss) | T4 ↑ (2–4 h exposure) | radioimmunossay kits | [41] |
| 0.4 and 0.8 mg Cd/L | 1 week | juvenile rainbow trout (Oncorhynchus mykiss) | T4 ↓ | radioimmunoassay kits | [41] |
| 25 mg CdCl2/L | 24, 48, or 96 h | tilapia (Oreochromis niloticus) | plasma T3 ↓ | immunoassay | [42] |
| CdCl2 | 7, 17 and 28 days | catfish (Clarias batrachus) | thyrotropin inactivation plasma thyroid hormones ↓ | / | [43] |
| 0, 0.5 and 2.5 mg Cd/L | 96 h | Chinese rare minnow (Gobiocypris rarus) larvae | whole-body of fish thyroid hormones ↓ (2.5 mg Cd/L) tireoglobuline ↑ | enzyme-linked immunosorbent assay (ELISA) | [44] |
| 10 and 25 μg Cd/L | 30 days | adults rainbow trout (Oncorhynchus mykiss) | plasma T4 ↓; T3 ↓ | commercial radioimmunoassay kits | [45] |
| 1 and 5 g μg Cd/L | 30 days | juvenile rainbow trout (Oncorhynchus mykiss) | no effects plasma T4; T3 | commercial radioimmunoassay kits | [45] |
| 200 ppm Cd (as CdCl2) | 35 days, via drinking water | rats, Wistar albino ♂ | relative thyroid weight ↑ serum TSH ↑ serum T4 ↓ | commercial radioimmunoassay kits | [46] |
| 50 mg Cd/L (as CdCl2) | 4 weeks | rats, Sprague-Dawley ♂ | serum T4 ↓, T3 ↓ serum TSH ↑ | commercial kits | [47] |
| 5, 10, 25, 50 or 100 ppm (as CdCl2) | 1 month, via drinking water | rats, Sprague-Dawley ♂ | plasma TSH ↑ (5, 25 and 100 ppm) | radioimmunoassay | [48] |
| 25 and 50 mg/L (CdCl2)equivalence is 1.5 and 3 mg CdCl2/kg bw/day | 30 days, via drinking water | rats, Sprague-Dawley ♂ | TSH ↑ (at 12:00 and 16:00 h with the 25 mg/L and at 08:00 h with the mg/L) | specific double antibody radioimmunoassay | [49] |
| 0.55 and 2.19 mg/L (as CdCl2) | 12 weeks, via drinking water | rats, albino ♂ | T4 ↓, T3 ↓, TSH ↓ | radioimmunoassay method | [50] |
| 5 and 50 mg Cd/L (as CdCl2) | 12 months | rats, Wistar ♀ | serum T4 ↓ (50 mg/L); no effects serum T3 and TSH | radioimmunologically | [51] |
| 0.3, 0.6, 1.25, 2.5, 5 and 10 mg Cd/kg bw/day | 28 days | rats, Wistar ♂ | T3 ↓, FT3 ↓, T4 ↓, FT4 ↓ (BMDL5 0.059 mg/kg bw/day for T3; BMDL5 0.141 mg/kg bw/day for FT3; BMDL5 0.365 mg/kg bw/day for T4; BMDL5 0.354 mg/kg bw/day for FT4) | electrochemiluminescent immunoassay (ECLIA) | [52] |
| 2.5, 7.5 and 15 mg Cd/kg bw/day | 28 days | rats, Wistar ♂ | T4 ↓, FT4 ↓, TSH below the limit of quantification | commercial tests, Elecsys analyser | [60] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buha, A.; Matovic, V.; Antonijevic, B.; Bulat, Z.; Curcic, M.; Renieri, E.A.; Tsatsakis, A.M.; Schweitzer, A.; Wallace, D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. Int. J. Mol. Sci. 2018, 19, 1501. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051501
Buha A, Matovic V, Antonijevic B, Bulat Z, Curcic M, Renieri EA, Tsatsakis AM, Schweitzer A, Wallace D. Overview of Cadmium Thyroid Disrupting Effects and Mechanisms. International Journal of Molecular Sciences. 2018; 19(5):1501. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051501
Chicago/Turabian StyleBuha, Aleksandra, Vesna Matovic, Biljana Antonijevic, Zorica Bulat, Marijana Curcic, Elisavet A. Renieri, Aristidis M. Tsatsakis, Amie Schweitzer, and David Wallace. 2018. "Overview of Cadmium Thyroid Disrupting Effects and Mechanisms" International Journal of Molecular Sciences 19, no. 5: 1501. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19051501








