“Sweet Flavonoids”: Glycosidase-Catalyzed Modifications
Abstract
:1. Introduction
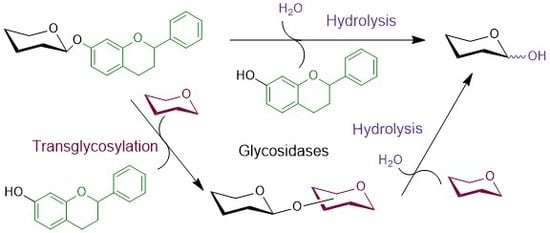
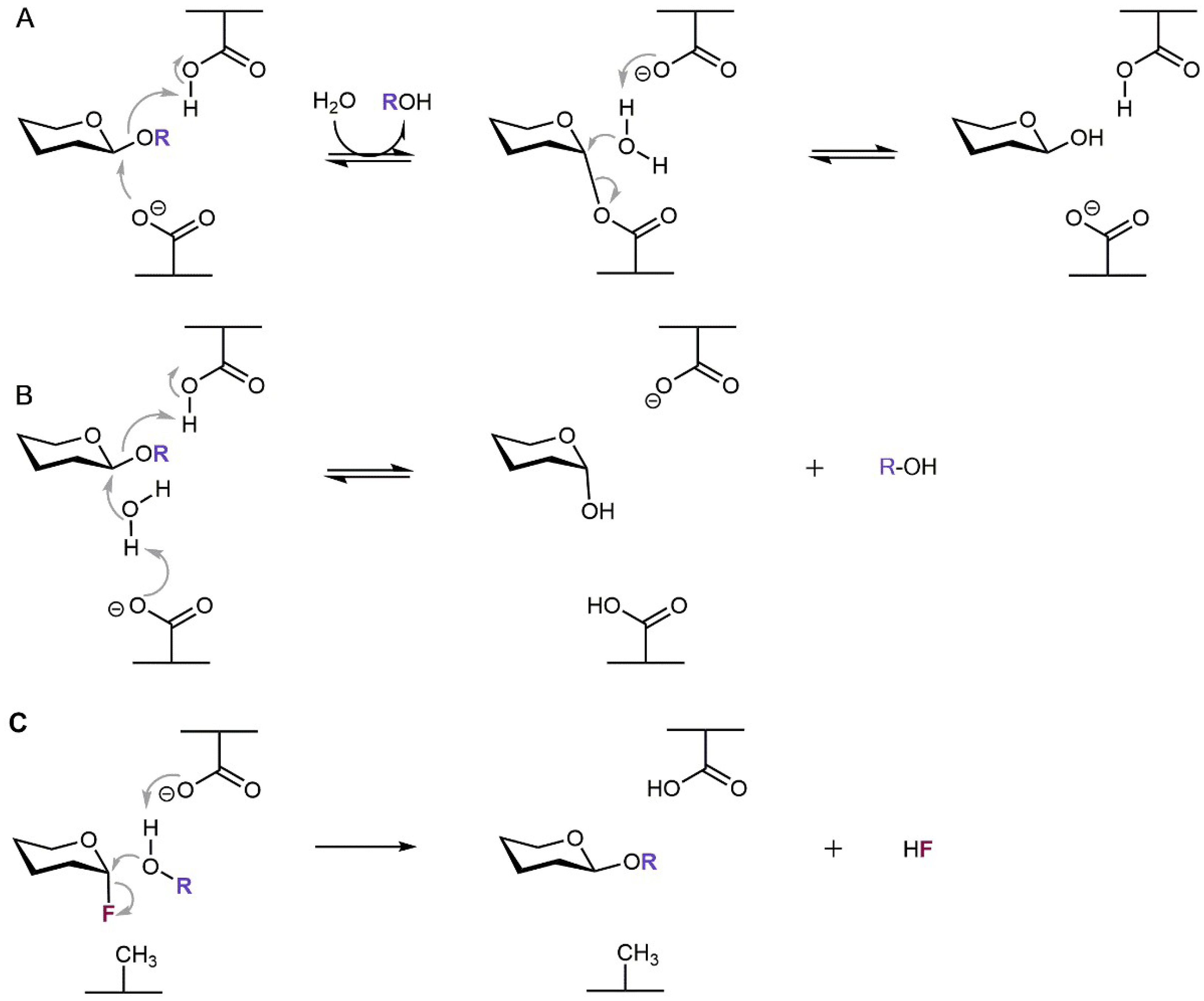
2. Glycosidase-Catalyzed (de)Glycosylations
3. Differences in Biophysical, Biochemical and Physiological Properties of Glycosylated and Deglycosylated Forms of Flavonoids
3.1. Solubility, Lipophilicity/Hydrophilicity and Stability
3.2. Bioavailability
3.3. Biological Activity
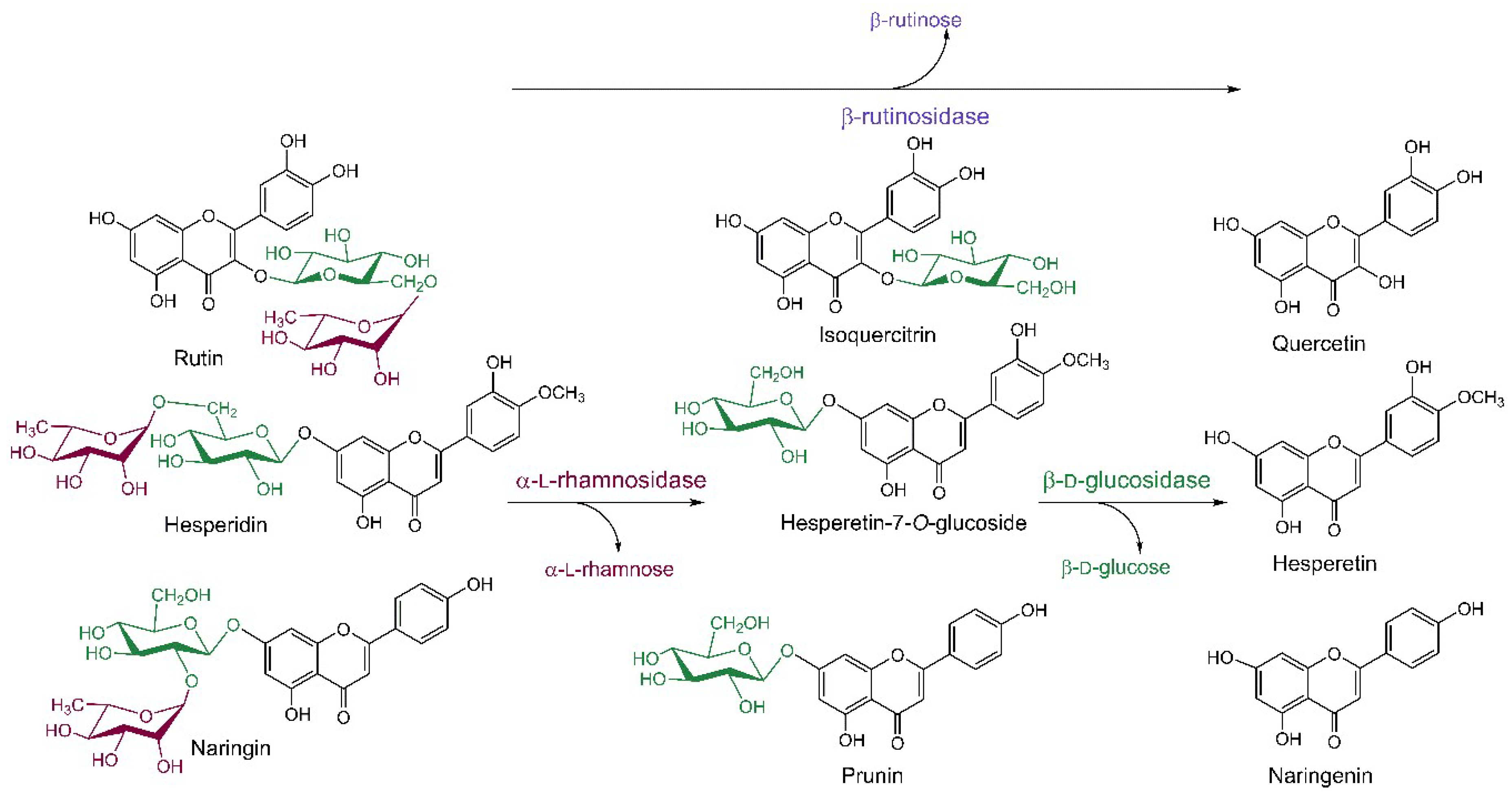
4. Selective Hydrolysis of Glycosylated Flavonoids
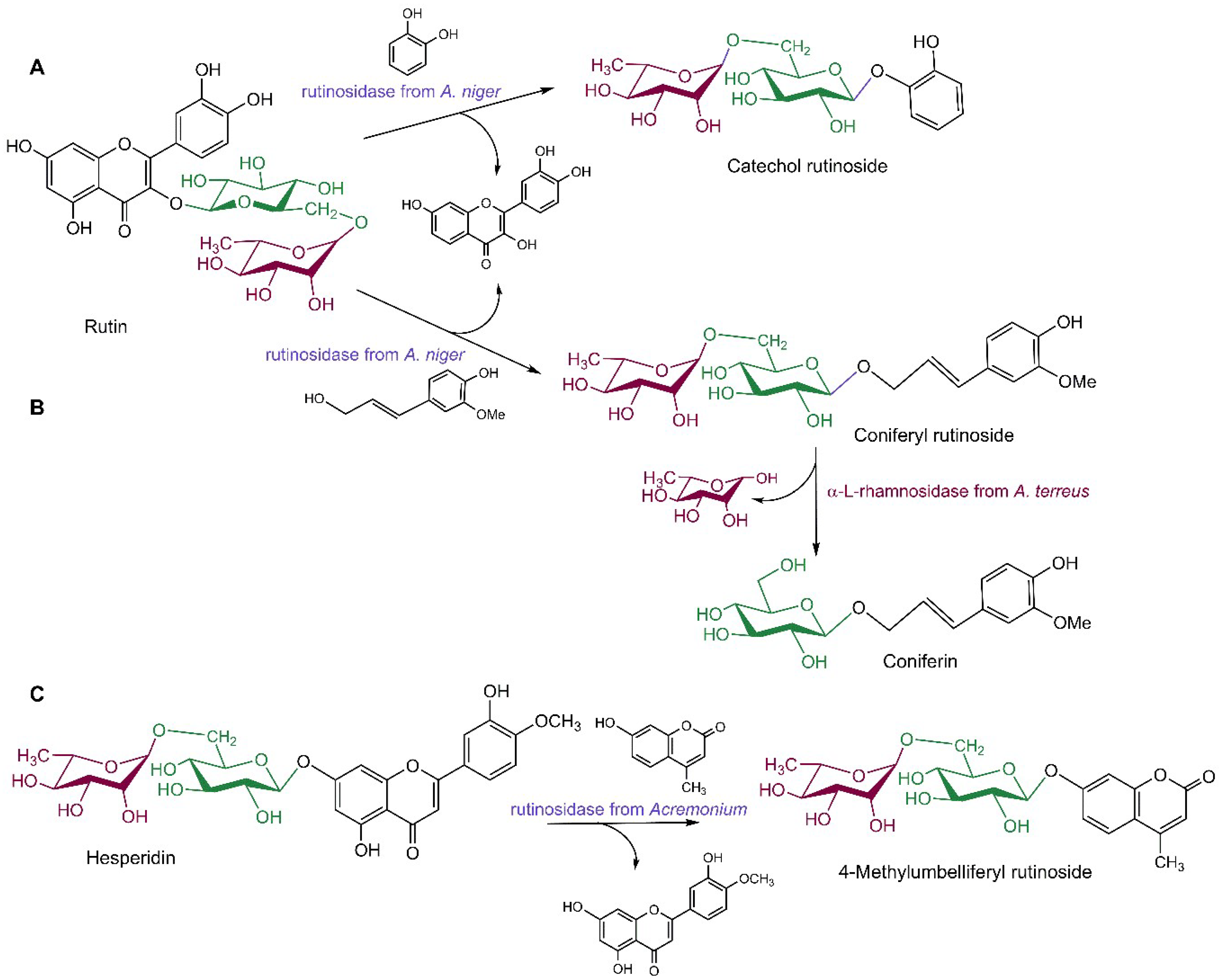
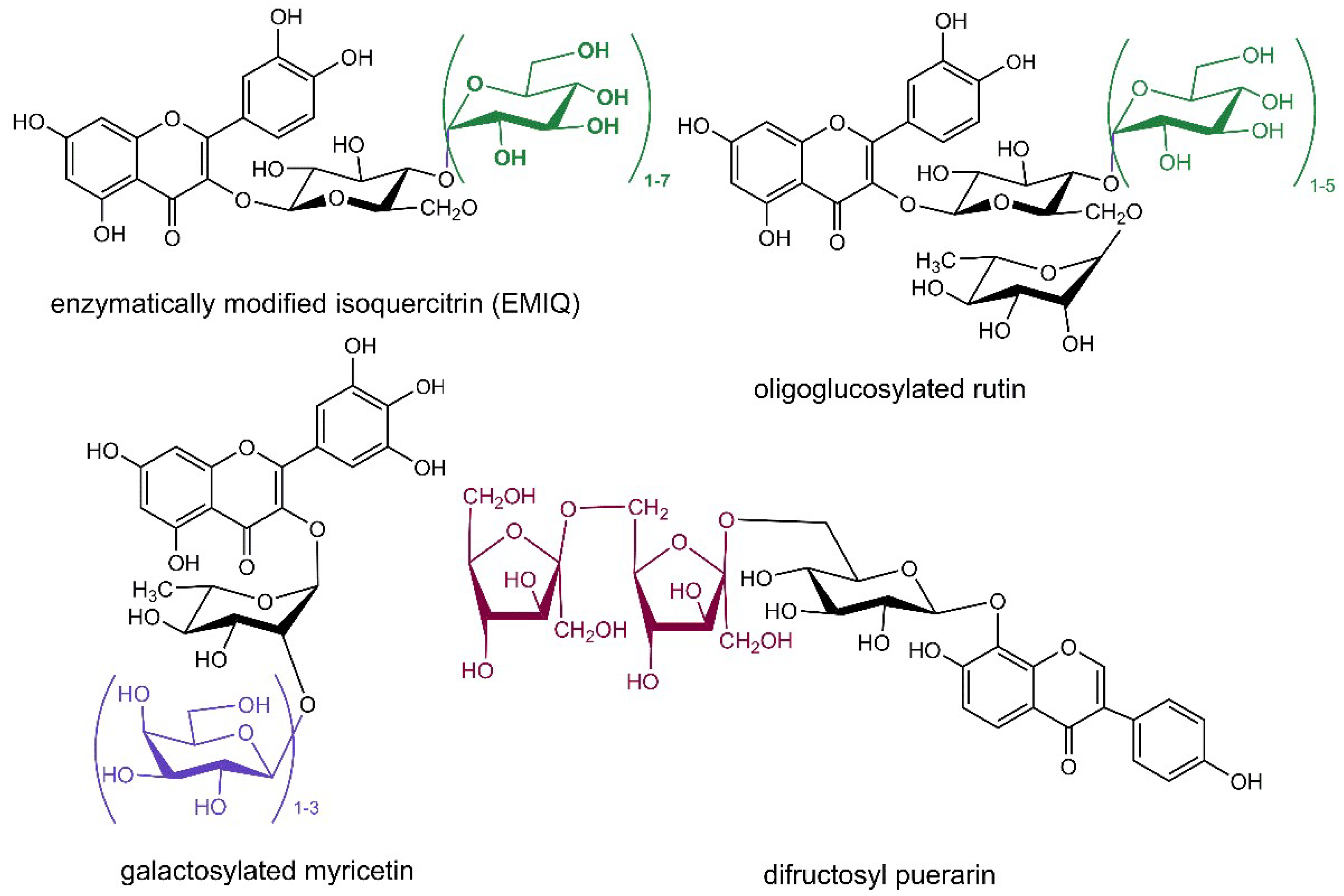
5. Glycosylation of Flavonoids by Glycosidases
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ara | α-l-Arabinopyranosyl |
| Araf | α-l-Arabinofuranosyl |
| CAZy | Carbohydrate-Active Enzymes database |
| EC | Enzyme Commission number |
| EMIQ | Enzymatically modified isoquercitrin |
| Gal | β-d-Galactopyranosyl |
| GH | Glycoside hydrolase |
| Glc | β-d-Glucopyranosyl |
| GRAS | Generally Recognized as Safe |
| HMG-CoA | 3-Hydroxy-3-methyl-glutaryl-coenzyme A |
| IUBMB | International Union of Biochemistry and Molecular Biology |
| Neo | Neohesperidosyl (2-O-α-l-rhamnopyranosyl-d-glucopyranosyl) |
| Rha | α-l-Rhamnopyranosyl (6-deoxy-α-l-mannopyranosyl) |
| Rha-Glc | 2-O-β-d-Glucopyranosyl-α-l-rhamnopyranosyl |
| Rha-Rha | 2-O-α-l-Rhamnopyranosyl-α-l-rhamnopyranosyl |
| Rha-Xyl | 2-O-β-d-Xylopyranosyl-α-l-rhamnopyranosyl |
| Rut | Rutinosyl (6-O-α-l-rhamnopyranosyl-β-d-glucopyranosyl) |
| UV | Ultraviolet |
References
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Edit. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Knaze, V.; Zamora-Ros, R. Polyphenols: Dietary assessment and role in the prevention of cancers. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, H.A.; Sohn, H.S.; Kim, J.W.; Choi, J.S. Potential of icariin metabolites from Epimedium koreanum Nakai as antidiabetic therapeutic agents. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Biler, M.; Biedermann, D.; Valentová, K.; Křen, V.; Kubala, M. Quercetin and its analogues: Optical and acido-basic properties. Phys. Chem. Chem. Phys. 2017, 19, 26870–26879. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Muzashvili, T.S.; Georgiev, M.I. Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol. Adv. 2014, 32, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Pozzo, T.; Liu, J.; Gulshan Ara, K.Z.; Turner, C.; Nordberg Karlsson, E. Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J. Agric. Food Chem. 2014, 62, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qi, T.; Xu, L.; Lu, L.; Xiao, M. Recent progress in the enzymatic glycosylation of phenolic compounds. J. Carbohyd. Chem. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, functions, and mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, J.; Yao, P.; Zhu, Y.; Men, Y.; Zeng, Y.; Yang, J.; Sun, Y. Exploiting the aglycon promiscuity of glycosyltransferase Bs-YjiC from Bacillus subtilis and its application in synthesis of glycosides. J. Biotechnol. 2017, 248, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gantt, R.W.; Goff, R.D.; Williams, G.J.; Thorson, J.S. Probing the aglycon promiscuity of an engineered glycosyltransferase. Angew. Chem. Int. Ed. 2008, 47, 8889–8892. [Google Scholar] [CrossRef] [PubMed]
- Bojarová, P.; Rosencrantz, R.R.; Elling, L.; Křen, V. Enzymatic glycosylation of multivalent scaffolds. Chem. Soc. Rev. 2013, 42, 4774–4797. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-pot synthesis of hyperoside by a three-enzyme cascade using a UDP-galactose regeneration system. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, B.G.; Ahn, J.H. Regioselective synthesis of flavonoid bisglycosides using Escherichia coli harboring two glycosyltransferases. Appl. Microbiol. Biotechnol. 2013, 97, 5275–5282. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Malla, S.; Simkhada, D.; Kim, B.G.; Sohng, J.K. Production of 3-O-xylosyl quercetin in Escherichia coli. Appl. Microbiol. Biotechnol. 2013, 97, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Slámová, K.; Bojarová, P. Engineered N-acetylhexosamine-active enzymes in glycoscience. Biochim. Biophys. Acta 2017, 1861, 2070–2087. [Google Scholar] [CrossRef] [PubMed]
- De Winter, K.; Dewitte, G.; Dirks-Hofmeister, M.E.; De Laet, S.; Pelantova, H.; Kren, V.; Desmet, T. Enzymatic glycosylation of phenolic antioxidants: Phosphorylase-mediated synthesis and characterization. J. Agric. Food Chem. 2015, 63, 10131–10139. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, L.S.; Pinuel, L.; Erra-Balsells, R.; Giudicessi, S.L.; Breccia, J.D. Transglycosylation specificity of Acremonium sp. α-rhamnosyl-β-glucosidase and its application to the synthesis of the new fluorogenic substrate 4-methylumbelliferyl-rutinoside. Carbohydr. Res. 2012, 347, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Šimčíková, D.; Kotik, M.; Weignerová, L.; Halada, P.; Pelantová, H.; Adamcová, K.; Křen, V. α-l-Rhamnosyl-β-d-glucosidase (rutinosidase) from Aspergillus niger: Characterization and synthetic potential of a novel diglycosidase. Adv. Synth. Catal. 2015, 357, 107–117. [Google Scholar] [CrossRef]
- Bojarová, P.; Křen, V. Glycosidases in carbohydrate synthesis: When organic chemistry falls short. Chimia (Aarau) 2011, 65, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Yadav, P.K.; Yadav, S.; Yadav, K.D.S. α-l-Rhamnosidase: A review. Process Biochem. 2010, 45, 1226–1235. [Google Scholar] [CrossRef]
- Weignerová, L.; Marhol, P.; Gerstorferová, D.; Křen, V. Preparatory production of quercetin-3-β-d-glucopyranoside using alkali-tolerant thermostable α-l-rhamnosidase from Aspergillus terreus. Bioresour. Technol. 2012, 115, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Vila-Real, H.; Alfaia, A.J.; Bronze, M.R.; Calado, A.R.; Ribeiro, M.H. Enzymatic synthesis of the flavone glucosides, prunin and isoquercetin, and the aglycones, naringenin and quercetin, with selective α-l-rhamnosidase and β-d-glucosidase activities of naringinase. Enzyme Res. 2011, 2011, 692618. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.H. Naringinases: Occurrence, characteristics, and applications. Appl. Microbiol. Biotechnol. 2011, 90, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Křen, V.; Valentová, K. Isoquercetin enzymatic production: A true story: Response to the paper “Zhua et al. [1]”. Mol. Catal. 2018. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef] [PubMed]
- Koshland, D.E. Stereochemistry and the mechanism of enzymatic reactions. Biol. Rev. 1953, 28, 416–436. [Google Scholar] [CrossRef]
- Vocadlo, D.J.; Davies, G.J. Mechanistic insights into glycosidase chemistry. Curr. Opin. Chem. Biol. 2008, 12, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Bojarová-Fialová, P.; Křen, V. Enzymatic approaches to O-glycoside introduction: Glycosidases A2-Kamerling, Hans. In Comprehensive Glycoscience; Elsevier: Oxford, UK, 2007; pp. 453–487. [Google Scholar]
- Fialová, P.; Weignerová, L.; Rauvolfová, J.; Přikrylová, V.; Pišvejcová, A.; Ettrich, R.; Kuzma, M.; Sedmera, P.; Křen, V.R. Hydrolytic and transglycosylation reactions of N-acyl modified substrates catalysed by β-N-acetylhexosaminidases. Tetrahedron 2004, 60, 693–701. [Google Scholar] [CrossRef]
- Armstrong, Z.; Withers, S.G. Synthesis of glycans and glycopolymers through engineered enzymes. Biopolymers 2013, 99, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, L.F.; Wang, Q.; Warren, R.A.J.; Withers, S.G. Glycosynthases: Mutant glycosidases for oligosaccharide synthesis. J. Am. Chem. Soc. 1998, 120, 5583–5584. [Google Scholar] [CrossRef]
- Malet, C.; Planas, A. From β-glucanase to β-glucansynthase: Glycosyl transfer to α-glycosyl fluorides catalyzed by a mutant endoglucanase lacking its catalytic nucleophile. FEBS Lett. 1998, 440, 208–212. [Google Scholar] [CrossRef]
- Williams, S.J.; Withers, S.G. Glycosyl fluorides in enzymatic reactions. Carbohyd. Res. 2000, 327, 27–46. [Google Scholar] [CrossRef]
- Slámová, K.; Krejzová, J.; Marhol, P.; Kalachova, L.; Kulik, N.; Pelantová, H.; Cvačka, J.; Křen, V. Synthesis of derivatized chitooligomers using transglycosidases engineered from the fungal GH20 β-N-acetylhexosaminidase. Adv. Synth. Catal. 2015, 357, 1941–1950. [Google Scholar] [CrossRef]
- Bojarová, P.; Křenek, K.; Kuzma, M.; Petrásková, L.; Bezouška, K.; Namdjou, D.-J.; Elling, L.; Křen, V. N-Acetylhexosamine triad in one molecule: Chemoenzymatic introduction of 2-acetamido-2-deoxy-β-d-galactopyranosyluronic acid residue into a complex oligosaccharide. J. Mol. Catal. B-Enzym. 2008, 50, 69–73. [Google Scholar] [CrossRef]
- Bojarová, P.; Slámová, K.; Křenek, K.; Gažák, R.; Kulik, N.; Ettrich, R.; Pelantová, H.; Kuzma, M.; Riva, S.; Adámek, D.; et al. Charged hexosaminides as new substrates for β-N-acetylhexosaminidase-catalyzed synthesis of immunomodulatory disaccharides. Adv. Synth. Catal. 2011, 353, 2409–2420. [Google Scholar] [CrossRef]
- Slámová, K.; Gažák, R.; Bojarová, P.; Kulik, N.; Ettrich, R.; Pelantová, H.; Sedmera, P.; Křen, V. 4-Deoxy-substrates for β-N-acetylhexosaminidases: How to make use of their loose specificity. Glycobiology 2010, 20, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Kitaoka, M.; Svensson, B.; Ohtsubo, K. Recent development of phosphorylases possessing large potential for oligosaccharide synthesis. Curr. Opin. Chem. Biol. 2013, 17, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Vrba, J.; Bancířová, M.; Ulrichová, J.; Křen, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, R.; Shimabayashi, H.; Moriwaki, M. Enzymatic production of highly soluble myricitrin glycosides using β-galactosidase. Biosci. Biotechnol. Biochem. 2006, 70, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Park, S.-H.; Shim, J.-H.; Lee, H.-S.; Tang, S.-Y.; Park, C.-S.; Park, K.-H. In vitro enzymatic modification of puerarin to puerarin glycosides by maltogenic amylase. Carbohyd. Res. 2004, 339, 2789–2797. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Shimizu, R.; Kanemaru, M.; Suzuki, Y.; Moriwaki, M.; Mizukami, H. Enzymatically modified isoquercitrin, α-oligoglucosyl quercetin 3-O-glucoside, is absorbed more easily than other quercetin glycosides or aglycone after oral administration in rats. Biol. Pharm. Bull. 2009, 32, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Huh, J.-Y.; Nam, S.-H.; Moon, S.-K.; Lee, S.-B. Enzymatic bioconversion of citrus hesperidin by Aspergillus sojae naringinase: Enhanced solubility of hesperetin-7-O-glucoside with in vitro inhibition of human intestinal maltase, HMG-CoA reductase, and growth of Helicobacter pylori. Food Chem. 2012, 135, 2253–2259. [Google Scholar] [CrossRef] [PubMed]
- Chebil, L.; Bouroukba, M.; Gaiani, C.; Charbonel, C.; Khaldi, M.; Engasser, J.M.; Ghoul, M. Elucidation of the kinetic behavior of quercetin, isoquercitrin, and rutin solubility by physicochemical and thermodynamic investigations. Ind. Eng. Chem. Res. 2013, 52, 1464–1470. [Google Scholar] [CrossRef]
- Chebil, L.; Humeau, C.; Anthoni, J.; Dehez, F.; Engasser, J.M.; Ghoul, M. Solubility of flavonoids in organic solvents. J. Chem. Eng. Data 2007, 52, 1552–1556. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental determination of octanol−water partition coefficients of quercetin and related flavonoids. J Agr. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Matsuda, N.; Kashino, Y.; Fujikura, Y.; Nakamura, T.; Kato, Y.; Shimizu, R.; Okuyama, S.; Tanaka, H.; Koda, T.; et al. α-Oligoglucosylation of a sugar moiety enhances the bioavailability of quercetin glucosides in humans. Arch. Biochem. Biophys. 2010, 501, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Shimizu, S.; Chujo, H.; Moon, J.-H.; Terao, J. Efficiency of absorption and metabolic conversion of quercetin and its glucosides in human intestinal cell line Caco-2. Arch. Biochem. Biophys. 2000, 384, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.H.; Hyun, Y.J.; Shim, J.; Hong, S.W.; Kim, D.H. Metabolism of rutin and poncirin by human intestinal microbiota and cloning of their metabolizing α-l-rhamnosidase from Bifidobacterium dentium. J. Microbiol. Biotechnol. 2015, 25, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of quercetin in humans with a focus on interindividual variation. Comp. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-β-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Cermak, R.; Landgraf, S.; Wolffram, S. The bioavailability of quercetin in pigs depends on the glycoside moiety and on dietary factors. J. Nutr. 2003, 133, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Vree, T.B.; Katan, M.B. Bioavailabilities of quercetin-3-glucoside and quercetin-4'-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Feng, Y.; Ouyang, H.; Yu, B.; Chang, Y.; Pan, G.; Dong, G.; Wang, T.; Gao, X. A sensitive LC-MS/MS method for simultaneous determination of six flavonoids in rat plasma: Application to a pharmacokinetic study of total flavonoids from mulberry leaves. J. Pharm. Biomed. Anal. 2013, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.W.; Zhang, L.; Liu, X.; Chen, X.H.; Bi, K.S. HPLC analysis and pharmacokinetic study of quercitrin and isoquercitrin in rat plasma after administration of Hypericum japonicum Thunb. extract. Biomed. Chromatogr. 2008, 22, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Y.; Su, D.; Gao, G.; Zhou, X.; Sun, L.; Ba, X.; Chen, X.; Bi, K. A sensitive LC-MS-MS method for simultaneous quantification of two structural isomers, hyperoside and isoquercitrin: Application to pharmacokinetic studies. Chromatographia 2011, 73, 353–359. [Google Scholar] [CrossRef]
- Li, Z.; Meng, F.; Zhang, Y.; Sun, L.; Yu, L.; Zhang, Z.; Peng, S.; Guo, J. Simultaneous quantification of hyperin, reynoutrin and guaijaverin in mice plasma by LC-MS/MS: Application to a pharmacokinetic study. Biomed. Chromatogr. 2016, 30, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Wolffram, S.; Blöck, M.; Ader, P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Kottra, G.; Daniel, H. Flavonoid glycosides are not transported by the human Na+/glucose transporter when expressed in Xenopus laevis oocytes, but effectively inhibit electrogenic glucose uptake. J. Pharm. Exp. Ther. 2007, 322, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Bellocco, E.; Barreca, D.; Lagana, G.; Leuzzi, U.; Tellone, E.; Ficarra, S.; Kotyk, A.; Galtieri, A. Influence of L-rhamnosyl-d-glucosyl derivatives on properties and biological interaction of flavonoids. Mol. Cell. Biochem. 2009, 321, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 16. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Siri, D.; Lazzaroni, R.; Duroux, J.L. A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: The specificity of the 3-OH site. Food Chem. 2006, 97, 679–688. [Google Scholar] [CrossRef]
- de Araujo, M.E.; Moreira Franco, Y.E.; Alberto, T.G.; Sobreiro, M.A.; Conrado, M.A.; Priolli, D.G.; Frankland Sawaya, A.C.; Ruiz, A.L.; de Carvalho, J.E.; de Oliveira Carvalho, P. Enzymatic de-glycosylation of rutin improves its antioxidant and antiproliferative activities. Food Chem. 2013, 141, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D.S. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert. Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Houlmont, J.P.; Vercruysse, K.; Perez, E.; Rico-Lattes, I.; Bordat, P.; Treilhou, M. Cosmetic use formulations containing pentyl rhamnoside and cetyl rhamnoside. Int. J. Cosmet. Sci. 2001, 23, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, Y.; Zhang, X.; Jiang, Z.; Zhu, Y.; Xiao, A.; Ni, H.; Chen, F. Expression and biochemical characterization of recombinant α-l-rhamnosidase r-Rha1 from Aspergillus niger JMU-TS528. Int. J. Biol. Macromol. 2016, 85, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Pišvejcová, A.; Křen, V.; Lama, M.; Riva, S. Generation of an α-l-rhamnosidase library and its application for the selective derhamnosylation of natural products. Biotechnol. Bioeng. 2004, 87, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Lee, Y.-B.; Bae, H.-A.; Huh, J.-Y.; Nam, S.-H.; Sohn, H.-S.; Lee, H.J.; Lee, S.-B. Purification and characterisation of Aspergillus sojae naringinase: The production of prunin exhibiting markedly enhanced solubility with in vitro inhibition of HMG-CoA reductase. Food Chem. 2011, 124, 234–241. [Google Scholar] [CrossRef]
- Yadav, S.; Yadava, S.; Yadav, K.D. α-l-Rhamnosidase selective for rutin to isoquercitrin transformation from Penicillium griseoroseum MTCC-9224. Bioorg. Chem. 2017, 70, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, A.E.; Romero, C.M.; Castro, G.R. A novel α-l-rhamnosidase with potential applications in citrus juice industry and in winemaking. Eur. Food Res. Technol. 2013, 237, 977–985. [Google Scholar] [CrossRef]
- De Lise, F.; Mensitieri, F.; Tarallo, V.; Ventimiglia, N.; Vinciguerra, R.; Tramice, A.; Marchetti, R.; Pizzo, E.; Notomista, E.; Cafaro, V.; et al. RHA-P: Isolation, expression and characterization of a bacterial α-l-rhamnosidase from Novosphingobium sp PP1Y. J. Mol. Catal. B-Enzym. 2016, 134, 136–147. [Google Scholar] [CrossRef]
- Izzo, V.; Tedesco, P.; Notomista, E.; Pagnotta, E.; di Donato, A.; Trincone, A.; Tramice, A. α-Rhamnosidase activity in the marine isolate Novosphingobium sp PP1Y and its use in the bioconversion of flavonoids. J. Mol. Catal. B-Enzym. 2014, 105, 95–103. [Google Scholar] [CrossRef]
- Puri, M.; Kaur, A.; Singh, R.S.; Schwarz, W.H.; Kaur, A. One-step purification and immobilization of His-tagged rhamnosidase for naringin hydrolysis. Process Biochem. 2010, 45, 451–456. [Google Scholar] [CrossRef]
- Trincone, A. Uncommon glycosidases for the enzymatic preparation of glycosides. Biomolecules 2015, 5, 2160–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jia, H.; Xi, M.; Xu, L.; Wu, S.; Li, X. Purification and characterization of a naringinase from a newly isolated strain of Bacillus amyloliquefaciens 11568 suitable for the transformation of flavonoids. Food Chem. 2017, 214, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Shiono, Y.; Koseki, T. Biochemical characterization of Aspergillus oryzae recombinant α-l-rhamnosidase expressed in Pichia pastoris. J. Biosci. Bioeng. 2017, 124, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, H.; Zhang, C.; Lu, M.; Piao, Y.; Ohba, M.; Tang, M.; Yuan, X.; Wei, S.; Wang, K.; et al. Aspergillus niger DLFCC-90 rhamnoside hydrolase, a new type of flavonoid glycoside hydrolase. Appl. Environ. Microbiol. 2012, 78, 4752–4754. [Google Scholar] [CrossRef] [PubMed]
- Markošová, K.; Weignerová, L.; Rosenberg, M.; Křen, V.; Rebroš, M. Upscale of recombinant α-l-rhamnosidase production by Pichia pastoris MutS strain. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Céliz, G.; Rodriguez, J.; Soria, F.; Daz, M. Synthesis of hesperetin 7-O-glucoside from flavonoids extracted from Citrus waste using both free and immobilized α-l-rhamnosidases. Biocatal. Agric. Biotechnol. 2015, 4, 335–341. [Google Scholar] [CrossRef]
- Rebroš, M.; Pilniková, A.; Šimčíková, D.; Weignerová, L.; Stloukal, R.; Křen, V.; Rosenberg, M. Recombinant α-l-rhamnosidase of Aspergillus terreus immobilization in polyvinylalcohol hydrogel and its application in rutin derhamnosylation. Biocatal. Biotransform. 2013, 31, 329–334. [Google Scholar] [CrossRef]
- Guan, C.J.; Ji, Y.J.; Hu, J.L.; Hu, C.N.; Yang, F.; Yang, G.E. Biotransformation of rutin using crude enzyme from Rhodopseudomonas palustris. Curr. Microbiol. 2017, 74, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lu, L.L.; Xiao, M. Cell surface engineering of α-l-rhamnosidase for naringin hydrolysis. Bioresour. Technol. 2012, 123, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Kumar, D.; Yadava, S.; Yadav, K. De-rhamnosylation of hesperidin to hesperitin-7-O-glucoside by alkali tolerant α-l-rhamnosidase from Fusarium poae MTCC-2086. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 1952–1968. [Google Scholar] [CrossRef]
- Xia, Q.; Xu, D.; Huang, Z.; Liu, J.; Wang, X.; Wang, X.; Liu, S. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia 2010, 81, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.-L.; Wu, X.-Y.; Yu, L.; Xia, R.; Sun, G.-X.; Wu, F.-A. Selective hydrolysis by commercially available hesperidinase for isoquercitrin production. J. Mol. Catal. B-Enzym. 2012, 81, 37–42. [Google Scholar] [CrossRef]
- Zhu, D.; Gong, A.; Xu, Y.; Kinfack Tsabing, D.a.; Wu, F.; Wang, J. Isoquercitrin production from rutin catalyzed by naringinase under ultrasound irradiation. J. Mol. Catal. B-Enzym. 2016, 134, 186–195. [Google Scholar] [CrossRef]
- Kim, D.Y.; Yeom, S.J.; Park, C.S.; Kim, Y.S. Effect of high hydrostatic pressure treatment on isoquercetin production from rutin by commercial α-l-rhamnosidase. Biotechnol. Lett. 2016, 38, 1775–1780. [Google Scholar] [CrossRef] [PubMed]
- Vila-Real, H.; Alfaia, A.J.; Calado, A.R.; Ribeiro, M.H.L. Improvement of activity and stability of soluble and sol–gel immobilized naringinase in co-solvent systems. J. Mol. Catal. B-Enzym. 2010, 65, 91–101. [Google Scholar] [CrossRef]
- Wang, J.; Gong, A.; Yang, C.F.; Bao, Q.; Shi, X.Y.; Han, B.B.; Wu, X.Y.; Wu, F.A. An effective biphase system accelerates hesperidinase-catalyzed conversion of rutin to isoquercitrin. Sci. Rep. 2015, 5, 8682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstorferová, D.; Fliedrová, B.; Halada, P.; Marhol, P.; Křen, V.; Weignerová, L. Recombinant α-l-rhamnosidase from Aspergillus terreus in selective trimming of rutin. Process. Biochem. 2012, 47, 828–835. [Google Scholar] [CrossRef]
- De Winter, K.; Šimčíková, D.; Schalck, B.; Weignerová, L.; Pelantová, H.; Soetaert, W.; Desmet, T.; Křen, V. Chemoenzymatic synthesis of α-l-rhamnosides using recombinant α-l-rhamnosidase from Aspergillus terreus. Bioresour. Technol. 2013, 147, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, L.; Pinuel, L.; Minig, M.; Breccia, J.D. Extracellular monoenzyme deglycosylation system of 7-O-linked flavonoid β-rutinosides and its disaccharide transglycosylation activity from Stilbella fimetaria. Arch. Microbiol. 2010, 192, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kawasaki, M.; Shiono, Y.; Koseki, T. A novel Aspergillus oryzae diglycosidase that hydrolyzes 6-O-α-l-rhamnosyl-β-d-glucoside from flavonoids. Appl. Microbiol. Biotechnol. 2018, 102, 3193–3201. [Google Scholar] [CrossRef] [PubMed]
- Neher, B.D.; Mazzaferro, L.S.; Kotik, M.; Oyhenart, J.; Halada, P.; Kren, V.; Breccia, J.D. Bacteria as source of diglycosidase activity: Actinoplanes missouriensis produces 6-O-α-l-rhamnosyl-β-d-glucosidase active on flavonoids. Appl. Microbiol. Biotechnol. 2016, 100, 3061–3070. [Google Scholar] [CrossRef] [PubMed]
- Piñuel, L.; Breccia, J.D.; Guisan, J.M.; Lopez-Gallego, F. Production of hesperetin using a covalently multipoint immobilized diglycosidase from Acremonium sp. DSM24697. J. Mol. Microbiol. Biotechnol. 2013, 23, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Piñuel, L.; Mazzaferro, L.S.; Breccia, J.D. Operational stabilization of fungal α-rhamnosyl-β-glucosidase by immobilization on chitosan composites. Process Biochem. 2011, 46, 2330–2335. [Google Scholar] [CrossRef]
- Mazzaferro, L.S.; Breccia, J.D. Quantification of hesperidin in citrus-based foods using a fungal diglycosidase. Food Chem. 2012, 134, 2338–2344. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.K.; Hong, S.H.; Shin, K.C.; Oh, D.K. Quercetin production from rutin by a thermostable β-rutinosidase from Pyrococcus furiosus. Biotechnol. Lett. 2012, 34, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Desmet, T.; Soetaert, W.; Bojarová, P.; Křen, V.; Dijkhuizen, L.; Eastwick-Field, V.; Schiller, A. Enzymatic glycosylation of small molecules: Challenging substrates require tailored catalysts. Chemistry 2012, 18, 10786–10801. [Google Scholar] [CrossRef] [PubMed]
- Bassanini, I.; Krejzová, J.; Panzeri, W.; Monti, D.; Křen, V.; Riva, S. A sustainable one-pot, two-enzyme synthesis of naturally occurring arylalkyl glucosides. ChemSusChem 2017, 10, 2040–2045. [Google Scholar] [CrossRef] [PubMed]
- Minig, M.; Mazzaferro, L.S.; Erra-Balsells, R.; Petroselli, G.; Breccia, J.D. α-Rhamnosyl-β-glucosidase-catalyzed reactions for analysis and biotransformations of plant-based foods. J. Agric. Food Chem. 2011, 59, 11238–11243. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Kim, H.H.; Seo, D.H.; Jung, J.H.; Park, J.H.; Baek, N.I.; Kim, M.J.; Yoo, S.H.; Cha, J.; Kim, Y.R.; et al. Biosynthesis of (+)-catechin glycosides using recombinant amylosucrase from Deinococcus geothermalis DSM 11300. Enzyme. Microb. Technol. 2011, 49, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Kitao, S.; Ariga, T.; Matsudo, T.; Sekine, H. The syntheses of catechin-glucosides by transglycosylation with leuconostoc mesenteroides sucrose phosphorylase. Biosci. Biotechnol. Biochem. 1993, 57, 2010–2015. [Google Scholar] [CrossRef]
- Gao, C.; Mayon, P.; MacManus, D.A.; Vulfson, E.N. Novel enzymatic approach to the synthesis of flavonoid glycosides and their esters. Biotechnol. Bioeng. 2000, 71, 235–243. [Google Scholar] [CrossRef]
- Chen, S.; Xing, X.-H.; Huang, J.-J.; Xu, M.-S. Enzyme-assisted extraction of flavonoids from Ginkgo biloba leaves: Improvement effect of flavonol transglycosylation catalyzed by Penicillium decumbens cellulase. Enzym. Microb. Technol. 2011, 48, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Davies, G.J.; Davis, B.G. A glycosynthase catalyst for the synthesis of flavonoid glycosides. Angew. Chem. Int. Ed. Engl. 2007, 46, 3885–3888. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, T.; Plaza, M.; Romero-García, J.; Faijes, M.; Karlsson, E.N.; Planas, A. Glycosynthases from Thermotoga neapolitana β-glucosidase 1A: A comparison of α-glucosyl fluoride and in situ-generated α-glycosyl formate donors. J. Mol. Catal. B-Enzym. 2014, 107, 132–139. [Google Scholar] [CrossRef]
- FDA. GRAS Notification-α-glycosyl isoquercitrin. Available online: http://wayback.archive-it.org/7993/20171031051756/https://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM269110.pdf (accessed on 25 September 2013).
- Sun, T.; Jiang, B.; Pan, B. Microwave accelerated transglycosylation of rutin by cyclodextrin glucanotransferase from Bacillus sp. SK13.002. Int. J. Mol. Sci. 2011, 12, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Park, S.-H.; Gao, C.; Park, K.-H.; Gu, L. Identification and antioxidative properties of transglycosylated puerarins synthesised by an archaeal maltogenic amylase. Food Chem. 2011, 124, 603–608. [Google Scholar] [CrossRef]
- Wu, X.; Chu, J.; Wu, B.; Zhang, S.; He, B. An efficient novel glycosylation of flavonoid by β-fructosidase resistant to hydrophilic organic solvents. Bioresour. Technol. 2013, 129, 659–662. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072126
Slámová K, Kapešová J, Valentová K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. International Journal of Molecular Sciences. 2018; 19(7):2126. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072126
Chicago/Turabian StyleSlámová, Kristýna, Jana Kapešová, and Kateřina Valentová. 2018. "“Sweet Flavonoids”: Glycosidase-Catalyzed Modifications" International Journal of Molecular Sciences 19, no. 7: 2126. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072126