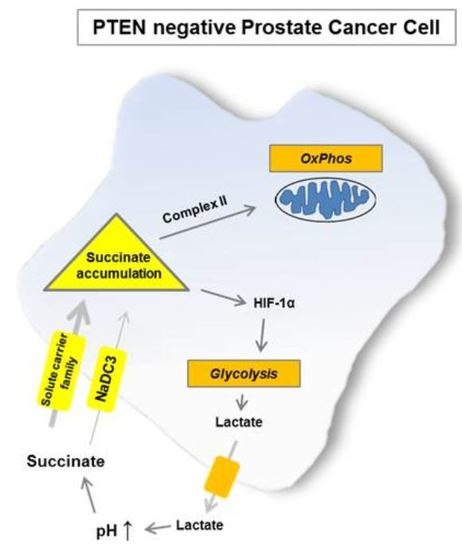
Succinate Accumulation Is Associated with a Shift of Mitochondrial Respiratory Control and HIF-1α Upregulation in PTEN Negative Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Loss of PTEN Is Associated with a Shift towards Succinate-Supported Mitochondrial Respiration and an Increase in Intracellular Succinate Levels
2.2. Elevated Succinate Levels Are Associated with Increased Lactate Production and Higher Expression of HIF-1α
2.3. Knockdown of NaDC3 Expression Is Not Sufficient to Prohibit Succinate Accumulation in Prostate Cancer Cells
2.4. Pharmacological Inhibition of Dicarboxylic Acid Transporters Attenuates Succinate Effects and Inhibits Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Growth Conditions
4.2. High-Resolution Respirometry (HRR)
4.3. Cell Viability Assay
4.4. Lactate Production
4.5. Proliferation Assay
4.6. Real-Time Quantitative PCR (qPCR)
4.7. Immunofluorescent Staining
4.8. Immunohistochemistry
4.9. Western Blotting
4.10. Transient Inhibition of NaDC3 with Small Interference RNA (siRNA)
4.11. Quantitative Analysis of Intracellular Succinate, Fumarate, and Lactate by Gas Chromatography-Mass Spectrometry (GC-MS)
4.12. 3D Hanging Drop Cell Culture
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PCa | Prostate cancer |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| PTEN | Phosphatase and Tensin homologe |
| NaDC3 | Sodium-dependent dicarboxylate transporter member 3 |
| siRNA | Small interference ribonucleic acid |
| siCtrl | Small interference control |
| OXPHOS | Oxidative phosphorylation |
| ATP | Adenosine triphosphate |
| CI:II, III, IV | Complex I, II, III, IV |
| ATCC | American Type Culture Collection |
| FCS | Fetal calf serum |
| DMEM | Dulbecco’s modified Eagle’s medium |
| RPMI | Roswell Park Memorial Institute |
| O2k | Oxygraph-2k |
| FCCP,CCCP | Carbonyl cyanide m-chloro phenyl hydrazine |
| LDH | Lactate dehydrogenase |
| NAD | Nicotinamide adenine dinucleotide |
| NADH | Nicotinamide adenine dinucleotide hydride |
| INT | P-iodonitrotetrazolium violet |
| INTH | P-iodonitrotetrazolium violet hydride |
| WST-1 | Water soluble tetrazolium-1 |
| DNA | Deoxyribonucleic acid |
| MOPS | 3-(N-morpholino) propane sulfonic acid |
| PBS | Phosphate buffered saline |
| AR | Androgen receptor |
| GAPDH | Glycerinaldehyd-3-phosphat-dehydrogenase |
| MSTFA | N-Methyl-N-trimethylsilyl-trifluoroacetamide |
| GC-MS | Gaschromatography-mass spectrometry |
| 3D | 3-dimensional |
| MiR05 | Mitochondrial respiration medium 05 |
| SEM | Standard error of the mean |
| WT | Wildtype |
| KO | Knockout |
| FAD | flavin adenine dinucleotide |
| FADH | flavin adenine dinucleotide hydride |
| S-ET | Succinate–electron transfer |
| FN(PGM) | Fatty acid oxidation, N-pathway (pyruvate, glutamate, malate) |
| FNS(PGM) | Fatty acid oxidation, N-pathway, S-pathway (pyruvate, glutamate, malate) |
| VceS | Viable cells Succinate |
| Pce | Permeabilized cells |
| Succ | Succinate |
| Dig | Digitonin |
| NH4OH | Ammonia solution |
| Na | Sodium |
| RNA | Ribonucleic acid |
| IHC | Immunohistochemistry |
| qPCR | Quantitative polymerase chain reaction |
| MCT1 | Monocarboxylate transporter 1 |
| mTOR | Mammalian target of rapamycin |
| PI3K | Phosphatidylinositol-4,5-bisphosphate 3-kinase |
| 4E-BP1 | Initiation factor 4E (eIF4E)-binding proteins |
| AMACR | p63/α-methylacyl-CoA racemase |
| TMA | Tissue microarray |
| kDA | Kilodalton |
| DMSO | Dimethyl sulfoxide |
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.; Prado-Garcia, H.; Sanchez-Garcia, F.J. Lactate contribution to the tumor microenvironment: Mechanisms, effects on immune cells and therapeutic relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J. Energy metabolism of cancer: Glycolysis versus oxidative phosphorylation. Oncol. Lett. 2012, 4, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Dueregger, A.; Schopf, B.; Eder, T.; Hofer, J.; Gnaiger, E.; Aufinger, A.; Kenner, L.; Perktold, B.; Ramoner, R.; Klocker, H.; et al. Differential utilization of dietary fatty acids in benign and malignant cells of the prostate. PLoS ONE 2015, 10, e0135704. [Google Scholar] [CrossRef] [PubMed]
- Schopf, B.; Schafer, G.; Weber, A.; Talasz, H.; Eder, I.E.; Klocker, H.; Gnaiger, E. Oxidative phosphorylation and mitochondrial function differ between human prostate tissue and cultured cells. FEBS J. 2016, 283, 2181–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; Pollard, P.J. Succinate: A new epigenetic hacker. Cancer Cell 2013, 23, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Zhunussova, A.; Sen, B.; Friedman, L.; Tuleukhanov, S.; Brooks, A.D.; Sensenig, R.; Orynbayeva, Z. Tumor microenvironment promotes dicarboxylic acid carrier-mediated transport of succinate to fuel prostate cancer mitochondria. Am. J. Cancer Res. 2015, 5, 1665–1679. [Google Scholar] [PubMed]
- Goo, C.K.; Lim, H.Y.; Ho, Q.S.; Too, H.P.; Clement, M.V.; Wong, K.P. PTEN/Akt signaling controls mitochondrial respiratory capacity through 4E-BP1. PLoS ONE 2012, 7, e45806. [Google Scholar] [CrossRef] [PubMed]
- Eder, T.; Weber, A.; Neuwirt, H.; Grunbacher, G.; Ploner, C.; Klocker, H.; Sampson, N.; Eder, I.E. Cancer-associated fibroblasts modify the response of prostate cancer cells to androgen and anti-androgens in three-dimensional spheroid culture. Int. J. Mol. Sci. 2016, 17, 1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, H.; Wu, F.; Zhang, Y.; Wang, J.; Zhao, L.; Guo, X.; Chang, L.J.; Zhang, Y.; You, M.J.; et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014, 8, 1461–1474. [Google Scholar] [CrossRef] [PubMed]
- Selak, M.A.; Armour, S.M.; MacKenzie, E.D.; Boulahbel, H.; Watson, D.G.; Mansfield, K.D.; Pan, Y.; Simon, M.C.; Thompson, C.B.; Gottlieb, E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell 2005, 7, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta 2016, 1857, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Yang, Z.H.; Fisher, G.; Tikishvili, E.; Stone, S.; Lanchbury, J.S.; Camacho, N.; Merson, S.; Brewer, D.; Cooper, C.S.; et al. Prognostic value of PTEN loss in men with conservatively managed localised prostate cancer. Br. J. Cancer 2013, 108, 2582–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueregger, A.; Heidegger, I.; Ofer, P.; Perktold, B.; Ramoner, R.; Klocker, H.; Eder, I.E. The use of dietary supplements to alleviate androgen deprivation therapy side effects during prostate cancer treatment. Nutrients 2014, 6, 4491–4519. [Google Scholar] [CrossRef] [PubMed]
- De Saedeleer, C.J.; Copetti, T.; Porporato, P.E.; Verrax, J.; Feron, O.; Sonveaux, P. Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS ONE 2012, 7, e46571. [Google Scholar] [CrossRef] [PubMed]
- Audet-Walsh, E.; Vernier, M.; Yee, T.; Laflamme, C.E.; Li, S.; Chen, Y.; Giguere, V. SREBF1 activity is regulated by an AR/mTOR nuclear axis in prostate cancer. Mol. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huwaimel, B.; Verma, K.; Miller, J.; Germain, T.M.; Kinarivala, N.; Pappas, D.; Brookes, P.S.; Trippier, P.C. Synthesis and antineoplastic evaluation of mitochondrial complex II (Succinate Dehydrogenase) inhibitors derived from Atpenin A5. ChemMedChem 2017, 12, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shestov, A.A.; Worth, A.J.; Nath, K.; Nelson, D.S.; Leeper, D.B.; Glickson, J.D.; Blair, I.A. Inhibition of mitochondrial complex II by the anticancer agent lonidamine. J. Biol. Chem. 2016, 291, 42–57. [Google Scholar] [CrossRef] [PubMed]
- Kluckova, K.; Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Neuzil, J. Mitochondrial complex II, a novel target for anti-cancer agents. Biochim. Biophys. Acta 2013, 1827, 552–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, X.Y.; Hou, K.; Chen, X.M.; Feng, Z.; Fu, B.; Wu, D. Subcellular localization signal analysis of high-affinity sodium-dependent dicarboxylate co-transporter protein. Prog. Biochem. Biophys. 2004, 31, 881–886. [Google Scholar]
- Chen, X.; Tsukaguchi, H.; Chen, X.Z.; Berger, U.V.; Hediger, M.A. Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter. J. Clin. Investig. 1999, 103, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Fei, Y.J.; Kekuda, R.; Yang-Feng, T.L.; Devoe, L.D.; Leibach, F.H.; Prasad, P.D.; Ganapathy, V. Structure, function, and genomic organization of human Na(+)-dependent high-affinity dicarboxylate transporter. Am. J. Physiol. Cell Physiol. 2000, 278, C1019–1030. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas. SLC13A3. Available online: https://www.proteinatlas.org/ENSG00000158296-SLC13A3/tissue (accessed on 16 April 2018).
- Pubchem. Compound Summary for CID 443130. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Mersalyl_acid#section=Drug-Indication (accessed on 16 April 2018).
- LaNoue, K.F.; Schoolwerth, A.C. Metabolite transport in mitochondria. Annu. Rev. Biochem. 1979, 48, 871–922. [Google Scholar] [CrossRef] [PubMed]
- Fonyo, A. SH-group reagents as tools in the study of mitochondrial anion transport. J. Bioenerg. Biomembr. 1978, 10, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.D.; Bhattacharya, P.; Wagner, S.; Tran, T.; Sailasuta, N. Hyperpolarized MR imaging: Neurologic applications of hyperpolarized metabolism. AJNR Am. J. Neuroradiol. 2010, 31, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, J.; Huang, J.; Powell, W.C.; Zhang, J.; Matusik, R.J.; Sangiorgi, F.O.; Maxson, R.E.; Sucov, H.M.; Roy-Burman, P. Generation of a prostate epithelial cell-specific CRE transgenic mouse model for tissue-specific gene ablation. Mech. Dev. 2001, 101, 61–69. [Google Scholar] [CrossRef]
- Wang, S.; Gao, J.; Lei, Q.; Rozengurt, N.; Pritchard, C.; Jiao, J.; Thomas, G.V.; Li, G.; Roy-Burman, P.; Nelson, P.S.; et al. Prostate-specific deletion of the murine PTEN tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003, 4, 209–221. [Google Scholar] [CrossRef]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar] [PubMed]
- Babson, A.L.; Babson, S.R. Kinetic colorimetric measurement of serum lactate dehydrogenase activity. Clin. Chem. 1973, 19, 766–769. [Google Scholar] [PubMed]
- Sampson, N.; Koziel, R.; Zenzmaier, C.; Bubendorf, L.; Plas, E.; Jansen-Durr, P.; Berger, P. ROS signaling by NOX4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Mol. Endocrinol. 2011, 25, 503–515. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, A.; Klocker, H.; Oberacher, H.; Gnaiger, E.; Neuwirt, H.; Sampson, N.; Eder, I.E. Succinate Accumulation Is Associated with a Shift of Mitochondrial Respiratory Control and HIF-1α Upregulation in PTEN Negative Prostate Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2129. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072129
Weber A, Klocker H, Oberacher H, Gnaiger E, Neuwirt H, Sampson N, Eder IE. Succinate Accumulation Is Associated with a Shift of Mitochondrial Respiratory Control and HIF-1α Upregulation in PTEN Negative Prostate Cancer Cells. International Journal of Molecular Sciences. 2018; 19(7):2129. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072129
Chicago/Turabian StyleWeber, Anja, Helmut Klocker, Herbert Oberacher, Erich Gnaiger, Hannes Neuwirt, Natalie Sampson, and Iris E. Eder. 2018. "Succinate Accumulation Is Associated with a Shift of Mitochondrial Respiratory Control and HIF-1α Upregulation in PTEN Negative Prostate Cancer Cells" International Journal of Molecular Sciences 19, no. 7: 2129. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072129