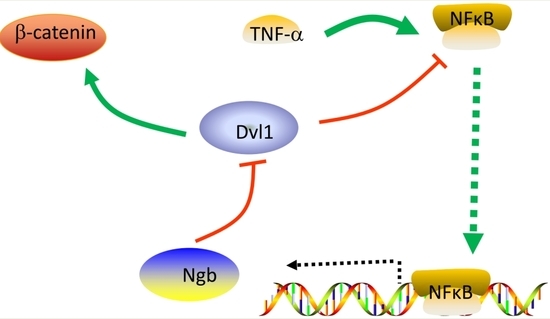
Neuroglobin Regulates Wnt/β-Catenin and NFκB Signaling Pathway through Dvl1
Abstract
:1. Introduction
2. Results
2.1. Ngb Interacts with Dvl1
2.2. Ngb Promotes the Proteasomal Degradation of Dvl1
2.3. Ngb Inhibits Wnt/β-Catenin Signaling Pathway via Dvl1
2.4. Ngb Enhances TNF-α-Induced NFκB Activation via Down-Regulating Dvl1
2.5. Ngb Protects SK-N-SH Cells from TNF-α-Induced Death
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Transfection
4.3. Luciferase Reporter Assays
4.4. Co-Immunoprecipitation and Western Blot
4.5. Immunocytochemistry
4.6. MTT Assays
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ngb | Neuroglobin |
| Dvl1 | Dishevelled-1 |
| Co-IP | co-immunoprecipitation |
| CHX | Heximide |
| TNF-α | tumor necrosis factor alpha |
| siRNA | small interfering RNA |
| NC siRNA | negative control small interfering RNA |
References
- Burmester, T.; Weich, B.; Reinhardt, S.; Hankeln, T. A vertebrate globin expressed in the brain. Nature 2000, 407, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Zhu, H.; Tejima, E.; Tsuji, K.; Murata, Y.; Atochin, D.N.; Huang, P.L.; Zhang, C.; Lo, E.H. Effects of neuroglobin overexpression on acute brain injury and long-term outcomes after focal cerebral ischemia. Stroke 2008, 39, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Yu, Z.; Zhao, G.; Xing, C.; Hayakawa, K.; Whalen, M.J.; Lok, J.M.; Lo, E.H.; Wang, X. Neuroglobin-overexpression reduces traumatic brain lesion size in mice. BMC Neurosci. 2012, 13, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.M.; Kelley, B.; Gregory, E.J.; Berman, N.E. Neuroglobin overexpression improves sensorimotor outcomes in a mouse model of traumatic brain injury. Neurosci. Lett. 2014, 577, 125–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.A.; Mao, X.O.; Banwait, S.; Jin, K.; Greenberg, D.A. Neuroglobin attenuates β-amyloid neurotoxicity in vitro and transgenic Alzheimer phenotype in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 19114–19119. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, Y.; Ren, C.; Lu, Y.; Gao, Y.; Zheng, X.; Zhang, C. The activity of recombinant human neuroglobin as an antioxidant and free radical scavenger. Proteins 2011, 79, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, N.; Li, Y.; Xu, J.; Wang, X. Neuroglobin overexpression inhibits oxygen-glucose deprivation-induced mitochondrial permeability transition pore opening in primary cultured mouse cortical neurons. Neurobiol. Dis. 2013, 56, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Antao, S.T.; Duong, T.T.; Aran, R.; Witting, P.K. Neuroglobin overexpression in cultured human neuronal cells protects against hydrogen peroxide insult via activating phosphoinositide-3 kinase and opening the mitochondrial K(ATP) channel. Antioxid. Redox Signal. 2010, 13, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, S.; Skommer, J.; Henty, K.; Birch, N.; Brittain, T. Neuroglobin protects nerve cells from apoptosis by inhibiting the intrinsic pathway of cell death. Apoptosis 2010, 15, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, N.; Liu, J.; Yang, K.; Wang, X. Neuroglobin, a novel target for endogenous neuroprotection against stroke and neurodegenerative disorders. Int. J. Mol. Sci. 2012, 13, 6995–7014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakasugi, K.; Nakano, T.; Morishima, I. Oxidized human neuroglobin acts as a heterotrimeric Gα protein guanine nucleotide dissociation inhibitor. J. Biol. Chem. 2003, 278, 36505–36512. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Mao, X.O.; Banwait, S.; DerMardirossian, C.M.; Bokoch, G.M.; Jin, K.; Greenberg, D.A. Regulation of hypoxic neuronal death signaling by neuroglobin. FASEB J. 2008, 22, 1737–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Liu, Q.R.; Xiong, X.X.; Liu, J.M.; Lai, X.J.; Cheng, C.; Pan, F.; Chen, Y.; Yu, S.B.; Yu, A.C.; et al. Neuroglobin promotes neurite outgrowth via differential binding to PTEN and Akt. Mol. Neurobiol. 2014, 49, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Xiong, Y.S.; Kong, F.L.; Qu, M.; Wang, Q.; Chen, X.Q.; Wang, J.Z.; Zhu, L.Q. Neuroglobin attenuates Alzheimer-like tau hyperphosphorylation by activating Akt signaling. J. Neurochem. 2012, 120, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, Y.B.; Sun, J.Y.; Xiang, Y.; Yang, J.; Dai, S.Y.; Zhang, X. Neuroglobin Attenuates Beta Amyloid-Induced Apoptosis Through Inhibiting Caspases Activity by Activating PI3K/Akt Signaling Pathway. J. Mol. Neurosci. 2016, 58, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Ai, Y.; Gong, H.; Chen, C.; Peng, Q.; Huang, L.; Wu, L.; Zhang, L.; Zhang, L. Neuroglobin Protects Rats from Sepsis-Associated Encephalopathy via a PI3K/Akt/Bax-Dependent Mechanism. J. Mol. Neurosci. 2017, 63, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; di Masi, A.; Leboffe, L.; Fiocchetti, M.; Nuzzo, M.T.; Brunori, M.; Marino, M. Neuroglobin: From structure to function in health and disease. Mol. Asp. Med. 2016, 52, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Palladino, P.; Scaglione, G.L.; Arcovito, A.; Maria Vitale, R.; Amodeo, P.; Vallone, B.; Brunori, M.; Benedetti, E.; Rossi, F. Neuroglobin-prion protein interaction: What’s the function? J. Pept. Sci. 2011, 17, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Wakasugi, K.; Nakano, T.; Morishima, I. Association of human neuroglobin with cystatin C, a cysteine proteinase inhibitor. Biochemistry 2004, 43, 5119–5125. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, T.; Tejero, J.; Chen, B.B.; Blood, A.B.; Frizzell, S.; Shapiro, C.; Tiso, M.; Hood, B.L.; Wang, X.; Zhao, X.; et al. 14-3-3 binding and phosphorylation of neuroglobin during hypoxia modulate six-to-five heme pocket coordination and rate of nitrite reduction to nitric oxide. J. Biol. Chem. 2011, 286, 42679–42689. [Google Scholar] [CrossRef] [PubMed]
- De Marinis, E.; Fiocchetti, M.; Acconcia, F.; Ascenzi, P.; Marino, M. Neuroglobin upregulation induced by 17β-estradiol sequesters cytocrome c in the mitochondria preventing H2O2-induced apoptosis of neuroblastoma cells. Cell Death Dis. 2013, 4, e508. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liu, N.; Wang, Y.; Li, X.; Wang, X. Identification of Neuroglobin-Interacting Proteins Using Yeast Two-Hybrid Screening. Neuroscience 2012, 200, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Ye, Y.; Wang, W.; Li, L. Dishevelled interacts with p65 and acts as a repressor of NF-κB-mediated transcription. Cell Res. 2010, 20, 1117–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wharton, K.A. Runnin’ with the Dvl: Proteins That Associate with Dsh/Dvl and Their Significance to Wnt Signal Transduction. Dev. Biol. 2003, 253, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.T.; Shah, K. Developmental biology: Signalling polarity. Nature 2002, 417, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Castro-Piedras, I.; Simmons, G.E.; Pruitt, K. Dishevelled: A masterful conductor of complex Wnt signals. Cell. Signal. 2018, 47, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Li, W.; Mao, X.; Winters, A.; Ryou, M.G.; Liu, R.; Greenberg, D.A.; Wang, N.; Jin, K.; Yang, S.H. Neuroglobin Overexpression Inhibits AMPK Signaling and Promotes Cell Anabolism. Mol. Neurobiol. 2016, 53, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.X.; Pan, F.; Chen, R.Q.; Hu, D.X.; Qiu, X.Y.; Li, C.Y.; Xie, X.Q.; Tian, B.; Chen, X.Q. Neuroglobin boosts axon regeneration during ischemic reperfusion via p38 binding and activation depending on oxygen signal. Cell Death Dis. 2018, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, C.; Chen, Y.G. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010, 22, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Taelman, V.F.; Dobrowolski, R.; Plouhinec, J.L.; Fuentealba, L.C.; Vorwald, P.P.; Gumper, I.; Sabatini, D.D.; De Robertis, E.M. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 2010, 143, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Peifer, M.; Polakis, P. Wnt signaling in oncogenesis and embryogenesis—A look outside the nucleus. Science 2000, 287, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Bielen, H.; Houart, C. The Wnt cries many: Wnt regulation of neurogenesis through tissue patterning, proliferation, and asymmetric cell division. Dev. Neurobiol. 2014, 74, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inestrosa, N.C.; Varela-Nallar, L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015, 359, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xie, Z.; Wang, J.; Li, M.; Jing, N.; Li, L. Nuclear factor of activated T cells (NFAT) proteins repress canonical Wnt signaling via its interaction with Dishevelled (Dvl) protein and participate in regulating neural progenitor cell proliferation and differentiation. J. Biol. Chem. 2011, 286, 37399–37405. [Google Scholar] [CrossRef] [PubMed]
- Haines, B.; Mao, X.; Xie, L.; Spusta, S.; Zeng, X.; Jin, K.; Greenberg, D.A. Neuroglobin expression in neurogenesis. Neurosci. Lett. 2013, 549, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, E.; Van Leuven, W.; Andre, D.; Quarta, A.; Reekmans, K.; Fransen, E.; Moens, L.; Hankeln, T.; Ponsaerts, P.; Dewilde, S. Loss of Neuroglobin Expression Alters Cdkn1a/Cdk6-Expression Resulting in Increased Proliferation of Neural Stem Cells. Stem Cells Dev. 2018, 27. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, E.A.M.; Butler, T.; Collier, L.; Collier, S.; Pennypacker, K.R. NF-κB protects neurons from ischemic injury after middle cerebral artery occlusion in mice. Brain Res. 2006, 1088, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Kaltschmidt, C. NF-κB in the Nervous System (vol 1745, pg 287, 2005). Cold Spring Harbor Perspect. Biol. 2010, 2. [Google Scholar] [CrossRef]
- Schneider, A.; Martin-Villalba, A.; Weih, F.; Vogel, J.; Wirth, T.; Schwaninger, M. NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat. Med. 1999, 5, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Meffert, M.K.; Chang, J.M.; Wiltgen, B.J.; Fanselow, M.S.; Baltimore, D. NF-κB functions in synaptic signaling and behavior. Nat. Neurosci. 2003, 6, 1072–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Zhou, D.; Bruce-Keller, A.J.; Kindy, M.S.; Mattson, M.P. Lack of the p50 subunit of nuclear factor-κB increases the vulnerability of hippocampal neurons to excitotoxic injury. J. Neurosci. 1999, 19, 8856–8865. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Deng, F.; Xing, Z.; Wu, Z.; Cen, B.; Xu, S.; Zhao, Z.; Nepomuceno, R.; Bhuiyan, M.I.H.; Sun, D.; et al. Long non-coding RNA C2dat1 regulates CaMKIIδ expression to promote neuronal survival through the NF-κB signaling pathway following cerebral ischemia. Cell Death Dis. 2016, 7, e2173. [Google Scholar] [CrossRef] [PubMed]
- Wajant, H.; Pfizenmaier, K.; Scheurich, P. Tumor necrosis factor signaling. Cell Death Differ. 2003, 10, 45–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Chen, C.; Wang, F.; Huang, W.; Liang, Z.; Xiao, Y.; Wei, K.; Wan, Z.; Hu, X.; Xiang, S.; et al. KCTD1 suppresses canonical Wnt signaling pathway by enhancing β-catenin degradation. PLoS ONE 2014, 9, e94343. [Google Scholar] [CrossRef] [PubMed]





| Name | Orientation | Sequence (5′-3′) |
|---|---|---|
| HA-Ngb | Forward | ACGCGTCGACCATGGAGCGCCCGGAGCC |
| HA-Ngb | Reverse | ATAAGAATGCGGCCGTTACTCGCCATCCCAGCCTCG |
| Myc-Dvl1 | Forward | ACGCGTCGACCATGGCGGAGACCAAGATTATCTA |
| Myc-Dvl1 | Reverse | ATAAGAATGCGGCCGCTCACATGATGTCCACGAAGA |
| Dvl1(1-250) | Forward | ACGCGTCGACCATGGCGGAGACCAAGATTATCTA |
| Dvl1(1-250) | Reverse | ATAAGAAT GCGGCCGCTCAGACGATGTTGAGGGACATG |
| Dvl1(1-378) | Forward | ACGCGTCGACCATGGCGGAGACCAAGATTATCTA |
| Dvl1(1-378) | Reverse | ATAAGAATGCGGCCGCTCACTCGTAGCGGGGCAGG |
| Dvl1(337-670) | Forward | ACGCGTCGACCAAGTGCTGGGACCCAAC |
| Dvl1(337-670) | Reverse | ATAAGAATGCGGCCGCTCACATGATGTCCACGAAGA |
| U6-siNgb | Forward | GATCCGGTGATGCTCGTGATTGATGCTTCAAGAGAGCATCAATCACGAGCATCACCTTTTTTA |
| U6-siNgb | Reverse | AGCTTAAAAAAGGTGATGCTCGTGATTGATGCTCTCTTGAAGCATCAATCACGAGCATCACCG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xun, Y.; Li, Z.; Tang, Y.; Yang, M.; Long, S.; Shu, P.; Li, J.; Xiao, Y.; Tang, F.; Wei, C.; et al. Neuroglobin Regulates Wnt/β-Catenin and NFκB Signaling Pathway through Dvl1. Int. J. Mol. Sci. 2018, 19, 2133. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072133
Xun Y, Li Z, Tang Y, Yang M, Long S, Shu P, Li J, Xiao Y, Tang F, Wei C, et al. Neuroglobin Regulates Wnt/β-Catenin and NFκB Signaling Pathway through Dvl1. International Journal of Molecular Sciences. 2018; 19(7):2133. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072133
Chicago/Turabian StyleXun, Yu, Zhen Li, Yingxin Tang, Manjun Yang, Shengwen Long, Pan Shu, Jiabing Li, Ye Xiao, Fen Tang, Chenxi Wei, and et al. 2018. "Neuroglobin Regulates Wnt/β-Catenin and NFκB Signaling Pathway through Dvl1" International Journal of Molecular Sciences 19, no. 7: 2133. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19072133