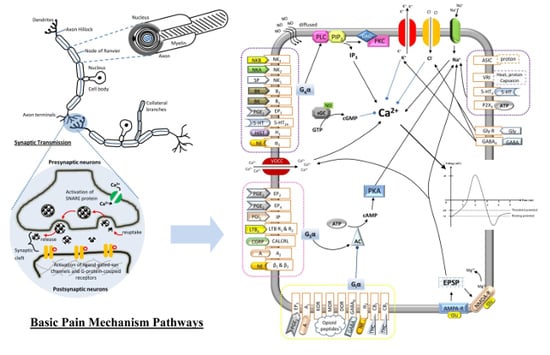
General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation
Abstract
:1. Introduction
2. Basic Mechanisms of Pain
2.1. Neurons
2.2. Axons
2.2.1. Group A
- Type Aα: both Type Ia and Ib of the sensory fibers from muscle spindle endings and Golgi tendon are grouped into this type. It is mainly used to determine the proprioceptive function.
- Type Aβ: it is a low-threshold, cutaneous, slow or fast adapting type of mechanoreceptors, and is a Type II afferent fiber from the stretch receptor [2]. The Aβ-fibers belong to laminae III and IV.
- Type Aγ: Type II afferent fibers from the stretch receptors.
- Type Aδ: it is well-known as the thermal and mechanical nociceptors that terminate in the rexed laminae I and V [3]. It is a Type III afferent fiber [4]. Aδ-fibers are also the smallest myelinated nerves and have a relatively fast conduction velocity of 30 m/s. The diameter of Aδ-fibers is about 2–5 µm, and is responsive towards short-lasting and pricking pain.
2.2.2. Group B
2.2.3. Group C
2.3. Action Potential
2.4. Synaptic Transmission
2.5. Route of Pain Transmission
3. Types of Pain
3.1. Nociceptive Pain
3.2. Neuropathic Pain
3.3. Inflammatory Pain
3.4. Arthritis
4. Hyperalgesia
5. Allodynia
6. Peripheral Sensitization
7. Central Sensitization
8. Neurogenic-Induced Inflammation
9. Major Types of Pain-Mediated Neurotransmitters
9.1. Tachykinins
9.2. Calcitonin Gene-Related Peptide
9.3. Bradykinin
9.4. Cytokines
9.5. Prostaglandins
9.6. Leukotriene B4
9.7. Proton
9.8. Adenosine Triphosphate
9.9. Nerve Growth Factor
9.10. Glutamate
9.11. γ-Aminobutyric Acid (GABA)
9.12. Opioid Peptides
9.13. Cannabinoids
9.14. Norepinephrine
10. Conclusions
Funding
Conflicts of Interest
References
- Woolf, C.J.; Bennett, G.J.; Doherty, M.; Dubner, R.; Kidd, B.; Koltzenburg, M.; Lipton, R.; Loeser, J.D.; Payne, R.; Torebjork, E. Towards a mechanism-based classification of pain? Pain 1998, 77, 227–229. [Google Scholar] [CrossRef]
- Hall, J.E. Guyton and Hall Textbook of Medical Physiology e-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2010. [Google Scholar]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Purves, D.; Augustine, G.; Fitzpatrick, D.; Hall, W.C.; LaMantia, A.; Mooney, R.; White, L.E. Neuroscience; Sinauer: Sunderland, MA, USA, 2018. [Google Scholar]
- Schmidt-Nielsen, K. Animal Physiology: Adaptation and Environment; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Ma, C.; Zhang, J.M. Animal Models of Pain; Humana Press: New York City, NY, USA, 2010. [Google Scholar]
- Waxman, S.G. The molecular pathophysiology of pain: Abnormal expression of sodium channel genes and its contributions to hyperexcitability of primary sensory neurons. Pain 1999, 82 (Suppl. 6), S133. [Google Scholar] [CrossRef]
- Narahashi, T.; Moore, J.W.; Scott, W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964, 47, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.K.; Starnes, T.W.; Deng, Q.; Huttenlocher, A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 2011, 480, 109. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.; Abbott, F.V. Techniques for assessing the effects of drugs on nociceptive responses. In Psychopharmacology; Springer: Berlin, Germany, 1989; pp. 145–216. [Google Scholar]
- Lu, R.; Bausch, A.E.; Kallenborn-Gerhardt, W.; Stoetzer, C.; Debruin, N.; Ruth, P.; Geisslinger, G.; Leffler, A.; Lukowski, R.; Schmidtko, A. Slack channels expressed in sensory neurons control neuropathic pain in mice. J. Neurosci. 2015, 35, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Merskey, H.E. Classification of chronic pain: Descriptions of chronic pain syndromes and definitions of pain terms. Pain 1986, 3, S1. [Google Scholar]
- Boyce-Rustay, J.M.; Honore, P.; Jarvis, M.F. Animal models of acute and chronic inflammatory and nociceptive pain. Methods Mol. Biol. 2010, 617, 41–55. [Google Scholar] [PubMed]
- Claar, D.; Hartert, T.V.; Peebles, R.S., Jr. The role of prostaglandins in allergic lung inflammation and asthma. Expert Rev. Respir. Med. 2015, 9, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Woodward, D.F.; Jones, R.L.; Narumiya, S. International union of basic and clinical pharmacology. Lxxxiii: Classification of prostanoid receptors, updating 15 years of progress. Pharmacol. Rev. 2011, 63, 471–538. [Google Scholar] [CrossRef] [PubMed]
- Back, M.; Powell, W.S.; Dahlen, S.E.; Drazen, J.M.; Evans, J.F.; Serhan, C.N.; Shimizu, T.; Yokomizo, T.; Rovati, G.E. Update on leukotriene, lipoxin and oxoeicosanoid receptors: Iuphar review 7. Br. J. Pharmacol. 2014, 171, 3551–3574. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.D.; Lam, D.; Taiwo, Y.O.; Donatoni, P.; Goetzl, E.J. Hyperalgesic properties of 15-lipoxygenase products of arachidonic acid. Proc. Natl. Acad. Sci. USA 1986, 83, 5331–5334. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.S.; Burch, R.L.; Crowder, R.J.; Lomb, D.J.; Schoell, M.C.; Straub, J.A.; Xie, L. Ngf deprivation-induced gene expression: After ten years, where do we stand? Prog. Brain Res. 2004, 146, 111–126. [Google Scholar] [PubMed]
- Riikonen, R.; Vanhala, R. Levels of cerebrospinal fluid nerve-growth factor differ in infantile autism and rett syndrome. Dev. Med. Child Neurol. 1999, 41, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Honore, P.; Zhong, C.; Gauvin, D.; Mikusa, J.; Hernandez, G.; Chandran, P.; Gomtsyan, A.; Brown, B.; Bayburt, E.K.; et al. Trpv1 receptors in the cns play a key role in broad-spectrum analgesia of trpv1 antagonists. J. Neurosci. 2006, 26, 9385–9393. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid vr1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.; Andreev, Y.A.; Kozlov, S. Acid-sensing ion channels and their modulators. Biochemistry 2014, 79, 1528–1545. [Google Scholar] [CrossRef] [PubMed]
- Sluka, K.A.; Winter, O.C.; Wemmie, J.A. Acid-sensing ion channels: A new target for pain and cns diseases. Curr. Opin. Drug Discov. Dev. 2009, 12, 693–704. [Google Scholar]
- Gironacci, M.M.; Carbajosa, N.A.L.; Goldstein, J.; Cerrato, B.D. Neuromodulatory role of angiotensin-(1–7) in the central nervous system. Clin. Sci. 2013, 125, 57–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llona, I.; Vavrek, R.; Stewart, J.; Huidobro-Toro, J.P. Identification of pre- and postsynaptic bradykinin receptor sites in the vas deferens: Evidence for different structural prerequisites. J. Pharmacol. Exp. Ther. 1987, 241, 608–614. [Google Scholar] [PubMed]
- McLean, P.G.; Ahluwalia, A.; Perretti, M. Association between kinin b1 receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J. Exp. Med. 2000, 192, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.; Perkins, M.; Dray, A. Kinins and kinin receptors in the nervous system. Neurochem. Int. 1995, 26, 1–16. [Google Scholar] [CrossRef]
- Burnstock, G. P2X receptors in sensory neurones. Br. J. Anaesth. 2000, 84, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Elmenhorst, D.; Meyer, P.T.; Winz, O.H.; Matusch, A.; Ermert, J.; Coenen, H.H.; Basheer, R.; Haas, H.L.; Zilles, K.; Bauer, A. Sleep deprivation increases a1 adenosine receptor binding in the human brain: A positron emission tomography study. J. Neurosci. 2007, 27, 2410–2415. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. Molecular physiology of p2x receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, H.E.; Schnermann, J.; Oldenburg, P.J.; Mustafa, S.J. Role of a1 adenosine receptors in regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Donkin, J.J.; Turner, R.J.; Hassan, I.; Vink, R. Substance p in traumatic brain injury. Prog. Brain Res. 2007, 161, 97–109. [Google Scholar] [PubMed]
- Gerard, N.P.; Eddy, R.L., Jr.; Shows, T.B.; Gerard, C. The human neurokinin a (substance k) receptor. Molecular cloning of the gene, chromosome localization, and isolation of cdna from tracheal and gastric tissues. J. Biol. Chem. 1990, 265, 20455–20462. [Google Scholar] [PubMed]
- Nagano, M.; Oishi, T.; Suzuki, H. Distribution and pharmacological characterization of primate nk-2 tachykinin receptor in the central nervous system of the rhesus monkey. Neurosci. Lett. 2011, 503, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Saria, A. The tachykinin nk1 receptor in the brain: Pharmacology and putative functions. Eur. J. Pharmacol. 1999, 375, 51–60. [Google Scholar] [CrossRef]
- Takeda, Y.; Chou, K.B.; Takeda, J.; Sachais, B.S.; Krause, J.E. Molecular cloning, structural characterization and functional expression of the human substance p receptor. Biochem. Biophys. Res. Commun. 1991, 179, 1232–1240. [Google Scholar] [CrossRef]
- Yip, J.; Chahl, L.A. Localization of nk1 and nk3 receptors in guinea-pig brain. Regul. Pept. 2001, 98, 55–62. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Marek, G.J. Serotonin, via 5-ht 2a receptors, increases epscs in layer v pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999, 825, 161–171. [Google Scholar] [CrossRef]
- Bortolozzi, A.; Díaz-Mataix, L.; Scorza, M.C.; Celada, P.; Artigas, F. The activation of 5-ht2a receptors in prefrontal cortex enhances dopaminergic activity. J. Neurochem. 2005, 95, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Férézou, I.; Cauli, B.; Hill, E.L.; Rossier, J.; Hamel, E.; Lambolez, B. 5-ht3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J. Neurosci. 2002, 22, 7389–7397. [Google Scholar] [CrossRef] [PubMed]
- Marek, G.J.; Wright, R.A.; Gewirtz, J.C.; Schoepp, D.D. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 2001, 105, 379–392. [Google Scholar] [CrossRef]
- Martin, P.; Waters, N.; Schmidt, C.; Carlsson, A.; Carlsson, M. Rodent data and general hypothesis: Antipsychotic action exerted through 5-ht2a receptor antagonism is dependent on increased serotonergic tone. J. Neural Transm. 1998, 105, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Shiroishi, M.; Weyand, S.; Tsujimoto, H.; Winter, G.; Katritch, V.; Abagyan, R.; Cherezov, V.; Liu, W.; Han, G.W.; et al. Structure of the human histamine h1 receptor complex with doxepin. Nature 2011, 475, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, R.E., Jr.; Zweig, A.; Shih, N.Y.; Siegel, M.I.; Egan, R.W.; Clark, M.A. Identification of two h3-histamine receptor subtypes. Mol. Pharmacol. 1990, 38, 610–613. [Google Scholar] [PubMed]
- Kleckner, N.W.; Dingledine, R. Requirement for glycine in activation of nmda-receptors expressed in xenopus oocytes. Science 1988, 241, 835–837. [Google Scholar] [CrossRef] [PubMed]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999, 51, 7–61. [Google Scholar] [PubMed]
- Furukawa, H.; Singh, S.K.; Mancusso, R.; Gouaux, E. Subunit arrangement and function in nmda receptors. Nature 2005, 438, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Moriyoshi, K.; Masu, M.; Ishii, T.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular cloning and characterization of the rat nmda receptor. Nature 1991, 354, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Platt, S.R. The role of glutamate in central nervous system health and disease—A review. Vet. J. 2007, 173, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale’s Pharmacology; Elsevier Health Sciences: London, UK, 2011. [Google Scholar]
- Saunders, C.; Limbird, L.E. Localization and trafficking of α2-adrenergic receptor subtypes in cells and tissues. Pharmacol. Ther. 1999, 84, 193–205. [Google Scholar] [CrossRef]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Arulmani, U.; Maassenvandenbrink, A.; Villalon, C.M.; Saxena, P.R. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur. J. Pharmacol. 2004, 500, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.; MacIntyre, I.; Morris, H.; Tippins, J.; Williams, T. Could the potent vasodilator calcitonin gene-related peptide be the mediator of the flare response in human skin? Regul. Pept. 1985, 13, 92. [Google Scholar] [CrossRef]
- Chen, L.J.; Zhang, F.G.; Li, J.; Song, H.X.; Zhou, L.B.; Yao, B.C.; Li, F.; Li, W.C. Expression of calcitonin gene-related peptide in anterior and posterior horns of the spinal cord after brachial plexus injury. J. Clin. Neurosci. 2010, 17, 87–91. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, J.; Uddman, R.; Kingman, T.A.; Edvinsson, L. Calcitonin gene-related peptide: Functional role in cerebrovascular regulation. Proc. Natl. Acad. Sci. USA 1986, 83, 5731–5735. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.G.; Mermod, J.J.; Amara, S.G.; Swanson, L.W.; Sawchenko, P.E.; Rivier, J.; Vale, W.W.; Evans, R.M. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 1983, 304, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Hyland, N.P.; Cryan, J.F. A gut feeling about GABA: Focus on GABAB receptors. Front. Pharmacol. 2010, 1, 124. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, L.E.; Russier, M.; Barbe, A.; Fritschy, J.M.; Bras, H. Differential organization of γ-aminobutyric acid type a and glycine receptors in the somatic and dendritic compartments of rat abducens motoneurons. J. Comp. Neurol. 2007, 504, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Gramsch, C.; Hollt, V.; Pasi, A.; Mehraein, P.; Herz, A. Immunoreactive dynorphin in human brain and pituitary. Brain Res. 1982, 233, 65–74. [Google Scholar] [CrossRef]
- James, I.F.; Chavkin, C.; Goldstein, A. Selectivity of dynorphin for κ opioid receptors. Life Sci. 1982, 31, 1331–1334. [Google Scholar] [CrossRef]
- Quock, R.M.; Burkey, T.H.; Varga, E.; Hosohata, Y.; Hosohata, K.; Cowell, S.M.; Slate, C.A.; Ehlert, F.J.; Roeske, W.R.; Yamamura, H.I. The δ-opioid receptor: Molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol. Rev. 1999, 51, 503–532. [Google Scholar] [PubMed]
- Baer, K.; Waldvogel, H.J.; Faull, R.L.; Rees, M.I. Localisation of glycine receptors in the human forebrain, brainstem, and cervical spinal cord: An immunohistochemical review. Front. Mol. Neurosci. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Naas, E.; Zilles, K.; Gnahn, H.; Betz, H.; Becker, C.M.; Schroder, H. Glycine receptor immunoreactivity in rat and human cerebral cortex. Brain Res. 1991, 561, 139–146. [Google Scholar] [CrossRef]
- Gong, J.-P.; Onaivi, E.S.; Ishiguro, H.; Liu, Q.-R.; Tagliaferro, P.A.; Brusco, A.; Uhl, G.R. Cannabinoid cb2 receptors: Immunohistochemical localization in rat brain. Brain Res. 2006, 1071, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Núñez, E.; Benito, C.; Pazos, M.R.; Barbachano, A.; Fajardo, O.; González, S.; Tolón, R.M.; Romero, J. Cannabinoid cb2 receptors are expressed by perivascular microglial cells in the human brain: An immunohistochemical study. Synapse 2004, 53, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Mechoulam, R. Is lipid signaling through cannabinoid 2 receptors part of a protective system? Prog. Lipid Res. 2011, 50, 193–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, N. Cannabinoid Receptor with an ’Identity Crisis’ Gets a Second Look; Nature Publishing Group: London, UK, 2015. [Google Scholar]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid cb2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Poyner, D.R.; Sexton, P.M.; Marshall, I.; Smith, D.M.; Quirion, R.; Born, W.; Muff, R.; Fischer, J.A.; Foord, S.M. International union of pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 2002, 54, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.C.; Tan, C.S.; Ch’ng, Y.S.; Ahmad, M.; Asmawi, M.Z.; Yam, M.F. Overview of antagonists used for determining the mechanisms of action employed by potential vasodilators with their suggested signaling pathways. Molecules 2016, 21, 495. [Google Scholar] [CrossRef] [PubMed]
- Loh, Y.C.; Tan, C.S.; Ch’ng, Y.S.; Yeap, Z.Q.; Ng, C.H.; Yam, M.F. Overview of the microenvironment of vasculature in vascular tone regulation. Int. J. Mol. Sci. 2018, 19, 120. [Google Scholar]
- Okamoto, K.; Imbe, H.; Morikawa, Y.; Itoh, M.; Sekimoto, M.; Nemoto, K.; Senba, E. 5-ht2a receptor subtype in the peripheral branch of sensory fibers is involved in the potentiation of inflammatory pain in rats. Pain 2002, 99, 133–143. [Google Scholar] [CrossRef]
- Loick, H.; Theissen, J. Die eicosanoide als mediatoren beim ards. Anästhesiologie Intensivmedizin·Notfallmedizin Schmerztherapie 1994, 29, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Crooks, S.W.; Stockley, R.A. Leukotriene b4. Int. J. Biochem. Cell Biol. 1998, 30, 173–178. [Google Scholar] [CrossRef]
- Price, M.P.; McIlwrath, S.L.; Xie, J.; Cheng, C.; Qiao, J.; Tarr, D.E.; Sluka, K.A.; Brennan, T.J.; Lewin, G.R.; Welsh, M.J. The drasic cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 2001, 32, 1071–1083. [Google Scholar] [CrossRef]
- Gould III, H.J. Complete freund’s adjuvant-induced hyperalgesia: A human perception. Pain 2000, 85, 301–303. [Google Scholar] [CrossRef]
- Montell, C.; Birnbaumer, L.; Flockerzi, V.; Bindels, R.J.; Bruford, E.A.; Caterina, M.J.; Clapham, D.E.; Harteneck, C.; Heller, S.; Julius, D.; et al. A unified nomenclature for the superfamily of trp cation channels. Mol. Cell 2002, 9, 229–231. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B.S. Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J. Nutr. 2000, 130 (Suppl. 4S). [Google Scholar] [CrossRef] [PubMed]
- Malmberg, A.B.; Brandon, E.P.; Idzerda, R.L.; Liu, H.; McKnight, G.S.; Basbaum, A.I. Diminished inflammation and nociceptive pain with preservation of neuropathic pain in mice with a targeted mutation of the type i regulatory subunit of camp-dependent protein kinase. J. Neurosci. 1997, 17, 7462–7470. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Maemura, K.; Kanbara, K.; Tamayama, T.; Hayasaki, H. Gaba and gaba receptors in the central nervous system and other organs. Int. Rev. Cytol. 2002, 213, 1–47. [Google Scholar] [PubMed]
- Pacher, P.; Bátkai, S.; Kunos, G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006, 58, 389–462. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Hohmann, A.G.; Martin, W.J.; Strangman, N.M.; Huang, S.M.; Tsou, K. The neurobiology of cannabinoid analgesia. Life Sci. 1999, 65, 665–673. [Google Scholar] [CrossRef]
- Demuth, D.G.; Molleman, A. Cannabinoid signalling. Life Sci. 2006, 78, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, J.L.; Ruckle, M.B.; Mayeux, P.R.; Prather, P.L. Agonist-directed trafficking of response by endocannabinoids acting at cb2 receptors. J. Pharmacol. Exp. Ther. 2005, 315, 828–838. [Google Scholar] [CrossRef] [PubMed]


 : Activate/Enhance production;
: Activate/Enhance production;  : Inhibit/Reduce production;
: Inhibit/Reduce production;  : Generate/Lead to.
: Generate/Lead to.
 : Activate/Enhance production;
: Activate/Enhance production;  : Inhibit/Reduce production;
: Inhibit/Reduce production;  : Generate/Lead to.
: Generate/Lead to.
| Neurotransmitters | Locations of Chemicals | Receptors: Mechanisms | Receptors’ Locations (Pre-/Post-Synaptically) | Agonists | Pharmacological Effects | Mediate Indirectly | References |
|---|---|---|---|---|---|---|---|
| Inflammatory Mediators | |||||||
| PGE2 & PGI2 (Eicosanoid) | CNS & PNS | EP1: PLC/IP3, DAG/PKC IP, EP2, EP3 & EP4: AC/cAMP/PKA | EP1–4: CNS, PNS (DRG of sensory neurons, mast cells, pulmonary veins, colon smooth muscle) IP: CNS (brain), PNS (thymus, VSMC, VEC, DRG in sensory neuron) (+/+) | EP1–4: PGE2, PGE1 IP: Prostacyclin | Excitatory (IP, EP1, EP2 & EP4)/inhibitory (EP3) | 1. Sensitize VR1 and SNS Nav receptors 2. Augment the release of SP, IL-2, histamine, 5-HT, bradykinin and CGRP | [15,16,17] |
| LTB4 (Eicosanoid) | PNS | LTB4-R1 & LTB4-R2: AC/cAMP/PKA or PLC | LTB4-R1 & LTB4-R2: PNS (nociceptive afferent neurons) (−/+) | LTB4 | Excitatory/ inhibitory | 1. Sensitize nociceptors 2. Recruit neutrophils to injury site 3. Promote the cytokines production | [18,19] |
| NGF (Neuropeptide) | CNS & PNS | TrkA: PI3/Ras | TrkA: PNS (primary afferent neurons) (−/+) | NGF, Neurotrophin | Excitatory | 1. Cause the mast cells degranulation2. Augment the release of 5-HT, histamine and itself | [20,21] |
| Proton | CNS & PNS | ASIC & VR1: Na+/K+ | ASIC: CNS (DH of spinal cord), PNS (sensory neurons) (−/+) VR1: CNS, PNS (dorsal root of primary sensory neurons) (+/+) | ASIC: Protons VR1: Heat, capsaicin and protons | Excitatory | 1. Enhance the release of BK, SP, CGRP, histamine and PGE2 | [22,23,24,25] |
| BK (Neuropeptide) | CNS (pituitary and hypothalamus) & PNS | B1 & B2: PLC/IP3, DAG/PKC | B1: CNS, PNS B2: CNS (cerebral cortex, hippocampus and spinal cord), PNS (nociceptive afferent neurons) (+/+) | BK | Excitatory (B1 & B2) | 1. Augment the release of PG, NGF and pro-inflammatory cytokines (IL-2). 2. Exert synergistic interaction with NGF and PG | [26,27,28,29] |
| ATP & Adenosine (Purine) | CNS & PNS | P2X3: Na+/ K+ A1 & A2: AC/cAMP/PKA | P2X3: CNS, PNS (nociceptive afferent neurons especially C-fibers) A1: CNS (basal forebrain), PNS (VSMC) A2: CNS (basal ganglia), PNS (vasculature, platelets) (+/+) | P2X3: ATP A1 & A2: Adenosine | Excitatory (P2X3 & A2)/inhibitory (A1) | 1. Sensitize the nociceptors 2. Enhance glutamate release | [30,31,32,33] |
| Tachykinins: SP, NKA and NKB (Neuropeptides) | CNS (predominant in DH of spinal cord) & PNS (from C-fibers) | NK1, NK2 & NK3: PLC/IP3, DAG/PKC | NK1: CNS (brainstem, spinal cord), PNS (VEC, muscle, immune cells)NK2: CNS (cingulated cortex, amygdale, prefrontal cortex)NK3: CNS, PNS (uterus, mesenteric vein, placenta) (−/+) | NK1: SP NK2: NKA NK3: NKB | Excitatory | 1. Activation of NOS and AA pathways for the release of NO and PGE2, respectively 2. Enhance the cAMP/PKA activities 3. Mediates neurogenic inflammation | [34,35,36,37,38,39] |
| 5-HT | CNS & PNS (platelet/GI) | 5-HT2A: PLC/IP3, DAG/PKC 5-HT3: Na+/ K+ | 5-HT2A: CNS (neocortex, olfactory tubercle), PNS (sensory neurons) 5-HT3: CNS (hippocampus, neocortical interneurons, amygdale), PNS (sensory neurons) (+/+) | 5-HT | Excitatory | 1. Exert synergistic interaction with NGF | [40,41,42,43,44] |
| Histamine (Monoamine) | CNS & PNS | H1: PLC/IP3, DAG/PKC | CNS, PNS (VSMC, VEC, sensory nerve) (+/−) | Histamine | Excitatory | 1. Exert synergistic interaction with NGF | [45,46] |
| Glutamate (Amino acid) | CNS (abundant) & PNS (in C-fibers) | AMPA-R & NMDA-R: Mg2+/Ca2+/Na+/K+ (EPSP) *NMDA-R need both glutamate/aspartate & co-exist of glycine to be activated | AMPA-R: CNS NMDA-R: CNS, PNS (nociceptive sensory neurons) (−/+) | AMPA-R: AMPA, glutamate NMDA-R: Glutamate, alanine, aspartate with co-exist of glycine [47] | Excitatory | [48,49,50,51] | |
| NE (Monoamine) | CNS & PNS | α1: PLC/IP3, DAG/PKC α2: AC/cAMP/PKA β: AC/cAMP/PKA | α1: CNS (brain), PNS (VSMC, GI, kidney) (−/+) α2: CNS (predominant), PNS (+/−) β: CNS (cerebral cortex), PNS (cardiac tissues) (+/+) | NE/Epinephrine/Isoprenline | Excitatory (α1 & β)/Inhibitory (α2) | [52,53] | |
| NO (Gasotransmitter) | CNS & PNS | sGC/cGMP | - | - | Excitatory/Inhibitory | 1. Recruited to the site of inflamed tissue | [54] |
| Non-inflammatory Mediators | |||||||
| CGRP (amino acid) | CNS (predominant in DH of spinal cord) & PNS | CALCRL: AC/cAMP/PKA | CALCRL: CNS (nucleus accumbens), PNS (cardiovascular, immune, respiratory, endocrine, primary afferent neurons) (−/+) | CGRP | Excitatory | 1. Synergistic with excitatory effect of SP | [55,56,57,58,59] |
| GABA (Amino acid) | CNS & PNS | GABAA: Cl−/K+ (IPSP) GABAB: AC/cAMP/PKA | GABAA: CNS, PNS (immune cells, endocrine tissues) GABAB: CNS, PNS (+/+) | GABA: muscimol, isoguvacine, gaboxadol, progabide | Inhibitory (GABAA & GABAB) | [60,61] | |
| Opioid Peptides (Neuropeptide) | CNS (hypothalamus, striatum, spinal cord, hippocampus) & PNS | KOR, MOR & DOR: AC/cAMP/PKA | KOR: CNS (PAG, RVM, brain, spinal cord), PNS (primary afferent neurons)MOR: CNS (PAG, RVM, cerebral cortex, amygdala, DH of spinal cord)DOR: CNS (basal ganglia, neocortical region) (+/+) | MOR: enkephalins & β-endorphins (high affinity) KOR: Dynorphins (high affinity) DOR: Enkephalins | Inhibitory | [62,63,64] | |
| Glycine (Amino acid) | CNS | GlyR: Cl− (IPSP) | CNS (−/+) | Glycine, β-alanine, Taurine | Inhibitory | [65,66] | |
| Cannabinoids (Lipid) | CNS (brain) & PNS | CB1 & CB2: AC/cAMP/PKA | CB1: CNS (brain and DH of spinal cord), PNS (lungs, kidneys, liver) (+/+) CB2: CNS (brainstem), PNS (immune cells, hematopoietic cells) (+/−) | Cannabinoids: THC, Anandamide, 2-Arachidonoylglycerol, 2-Arachidonyl glyceryl ether, N-Arachidonoyl dopamine, Virodhamine | Inhibitory (CB1 & CB2) | 1. Prevent the mast cells degranulation and the release of pro-inflammatory mediators | [67,68,69,70,71] |
| Intracellular Effectors | ||
|---|---|---|
| G-protein-coupled receptors (Metabotropic) | ||
| PLC/IP3, DAG/PKC | Gqα-protein-coupled receptors | Excitatory |
| Inhibit NE release, Inhibit AC/cAMP/PKA | Giα-protein-coupled receptors | Inhibitory |
| Activate AC/cAMP/PKA | Gsα-protein-coupled receptors | Excitatory |
| sGC/cGMP | NO-signaling cascade | Inhibitory |
| Ligand-gated ion channels (Ionotropic) | ||
| Cl− | GABAA | Inhibitory |
| Na+ | SNS Nav | Excitatory |
| Ca2+ | VOCC | Excitatory |
| K+ | Kv | Inhibitory |
| Protons (H+) | ASIC, VR1 | Excitatory |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19082164
Yam MF, Loh YC, Tan CS, Khadijah Adam S, Abdul Manan N, Basir R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. International Journal of Molecular Sciences. 2018; 19(8):2164. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19082164
Chicago/Turabian StyleYam, Mun Fei, Yean Chun Loh, Chu Shan Tan, Siti Khadijah Adam, Nizar Abdul Manan, and Rusliza Basir. 2018. "General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation" International Journal of Molecular Sciences 19, no. 8: 2164. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19082164