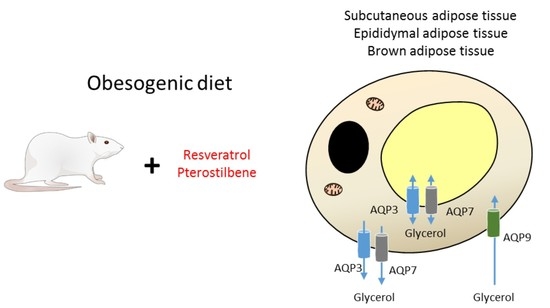
Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue
Abstract
:1. Introduction
2. Results
2.1. Adipose Tissue Weights
2.2. Serum Triglyceride Concentration
2.3. Adipocyte Number and Size Determination
2.4. Gene and Protein AQP Expressions in Epididymal and Subcutaneous White Adipose Tissues
2.5. Gene and Protein AQP Expressions in Brown Adipose Tissue
3. Discussion
4. Material and Methods
4.1. Animals, Diets and Experimental Design
4.2. Serum Triglyceride Concentration
4.3. DNA Extraction, Adipocyte Size, and Number Determination
4.4. Extraction and Analysis of RNA and Semi-Quantification by Reverse Transcription-Polymerase Chain Reaction (Real Time RT-PCR)
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prudente, S.; Flex, E.; Morini, E.; Turchi, F.; Capponi, D.; De Cosmo, S.; Tassi, V.; Guida, V.; Avogaro, A.; Folli, F.; et al. A functional variant of the adipocyte glycerol channel aquaporin 7 gene is associated with obesity and related metabolic abnormalities. Diabetes 2007, 56, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Catalan, V.; Gomez-Ambrosi, J.; Fruhbeck, G. Aquaglyceroporins serve as metabolic gateways in adiposity and insulin resistance control. Cell Cycle 2011, 10, 1548–1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gena, P.; Mastrodonato, M.; Portincasa, P.; Fanelli, E.; Mentino, D.; RodrÃguez, A.; Marinelli, R.A.; Brenner, C.; Fruhbeck, G.; Svelto, M.; et al. Liver glycerol permeability and aquaporin-9 are dysregulated in a murine model of non-alcoholic fatty liver disease. PLoS ONE 2013, 8, e78139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.; Gena, P.; Mendez-Gimenez, L.; Rosito, A.; Valenti, V.; Rotellar, F.; Sola, I.; Moncada, R.; Silva, C.; Svelto, M.; et al. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int. J. Obes. 2014, 38, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Laforenza, U.; Bottino, C.; Gastaldi, G. Mammalian aquaglyceroporin function in metabolism. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Olswang, Y.; Cassuto, H.; Blum, B.; Croniger, C.M.; Kalhan, S.C.; Tilghman, S.M.; Hanson, R.W. Glyceroneogenesis and the triglyceride/fatty acid cycle. J. Biol. Chem. 2003, 278, 30413–30416. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.S.; Hirsch, J.; Drewnowski, A.; Sullivan, A.C.; Johnson, P.R.; Cohn, C.K. Glycerol kinase activity in adipose tissue of obese rats and mice: Effects of diet composition. J. Nutr. 1983, 113, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, K.; Tauber, J.W.; Sigel, B.; Bombeck, C.T.; Jeffay, H. Glycerokinase activity in human adipose tissue as related to obesity. Int. J. Obes. 1984, 8, 609–622. [Google Scholar] [PubMed]
- Kaplan, M.L.; Leveille, G.A. Development of lipogenesis and insulin sensitivity in tissues of the ob/ob mouse. Am. J. Physiol. 1981, 240, E101–E107. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Kuriyama, H.; Funahashi, T.; Shimomura, I.; Kihara, S.; Ouchi, N.; Nishida, M.; Nishizawa, H.; Matsuda, M.; Takahashi, M.; et al. Aquaporin adipose, a putative glycerol channel in adipocytes. J. Biol. Chem. 2000, 275, 20896–20902. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Sohara, E.; Rai, T.; Ikawa, M.; Okabe, M.; Sasaki, S.; Uchida, S.; Verkman, A.S. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: Adipocyte glycerol permeability as a novel regulator of fat accumulation. J. Biol. Chem. 2005, 280, 15493–15496. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Moreno, N.R.; Balaguer, I.; Mendez-Gimenez, L.; Becerril, S.; Catalan, V.; Gomez-Ambrosi, J.; Portincasa, P.; Calamita, G.; Soveral, G.; et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Sci. Rep. 2015, 5, 12067. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Kubota, M.; Iguchi, K.; Usui, S.; Kiho, T.; Hirano, K. Membrane trafficking of aquaporin 3 induced by epinephrine. Biochem. Biophys. Res. Commun. 2008, 373, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Fernandez-Quintela, A.; Arias, N.; Portillo, M.P. Resveratrol: Anti-obesity mechanisms of action. Molecules 2014, 19, 18632–18655. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintela, A.; Carpene, C.; Fernandez, M.; Aguirre, L.; Milton-Laskibar, I.; Contreras, J.; Portillo, M.P. Anti-obesity effects of resveratrol: Comparison between animal models and humans. J. Physiol. Biochem. 2016, 73, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Quintela, A.; Milton-Laskibar, I.; Gonzalez, M.; Portillo, M.P. Antiobesity effects of resveratrol: Which tissues are involved? Ann. N. Y. Acad. Sci. 2017, 1403, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispo. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.H.; McCormick, D.L. Pharmacokinetics, oral bioavailability and metabolic profile ofd resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2010, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Zorita, S.; Fernandez-Quintela, A.; Lasa, A.; Aguirre, L.; Rimando, A.M.; Portillo, M.P. Pterostilbene, a dimethyl ether derivative of resveratrol, reduces fat accumulation in rats fed an obesogenic diet. J. Agric. Food. Chem. 2014, 62, 8371–8378. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Wu, J.C.; Ho, C.T.; Lai, C.S. Antiobesity molecular mechanisms of action: Resveratrol and pterostilbene. Biofactors 2018, 44, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Milton-Laskibar, I.; Hijona, E.; Bujanda, L.; Rimando, A.M.; Portillo, M.P. Effects of pterostilbene in brown adipose tissue from obese rats. J. Physiol. Biochem. 2016, 73, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Boque, N.; de la Iglesia, R.; de la Garza, A.L.; Milagro, F.I.; Olivares, M.; Banuelos, O.; Soria, A.C.; Rodriguez-Sanchez, S.; Martinez, J.A.; Campion, J. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol. Nutr. Food Res. 2013, 57, 1473–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Seo, J.S. Lycopene supplementation suppresses oxidative stress induced by a high fat diet in gerbils. Nutr. Res. Pract. 2013, 7, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Kim, H.R.; Kim, J.H.; Om, A.S. Beneficial effects of Allium sativum L. stem extract on lipid metabolism and antioxidant status in obese mice fed a high-fat diet. J. Sci. Food Agric. 2013, 93, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, S.; Unnikrishnan, A.; Ranjit, R.; Qaisar, R.; Pharaoh, G.; Matyi, S.; Deepa, S.S. A fish oil diet induces mitochondrial uncoupling and mitochondrial unfolded protein response in epididymal white adipose tissue of mice. Free Radic. Biol. Med. 2017, 108, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Funahashi, T.; Hibuse, T.; Nagasawa, A.; Kishida, K.; Kuriyama, H.; Nakamura, T.; Kihara, S.; Shimomura, I.; Matsuzawa, Y. Adaptation to fasting by glycerol transport through aquaporin 7 in adipose tissue. Proc. Natl. Acad. Sci. USA 2004, 101, 17801–17806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Priego, T.; Sanchez, J.; Rodriguez, A.M.; Palou, A.; Pico, C. Gene expression patterns in visceral and subcutaneous adipose depots in rats are linked to their morphologic features. Cell. Physiol. Biochem. 2009, 24, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Ceperuelo-Mallafre, V.; Lecube, A.; Hernandez, C.; Chacon, M.R.; Fort, J.M.; Gallart, L.; Baena-Fustegueras, J.A.; Simo, R.; Vendrell, J. Gene expression of paired abdominal adipose AQP7 and liver AQP9 in patients with morbid obesity: Relationship with glucose abnormalities. Metabolism 2009, 58, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, M.T.; Lebeck, J.; Rojek, A.; Praetorius, J.; Fuchtbauer, E.M.; Frøkiær, J.; Nielsen, S. AQP7 is localized in capillaries of adipose tissue, cardiac and striated muscle: Implications in glycerol metabolism. American J. Physiol. Renal. Physiol. 2007, 292, F956–F965. [Google Scholar] [CrossRef] [PubMed]
- Agha, G.; Houseman, E.A.; Kelsey, K.T.; Eaton, C.B.; Buka, S.L.; Loucks, E.B. Adiposity is associated with DNA methylation profile in adipose tissue. Int. J. Epidemiol. 2014, 44, 1277–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robelin, J. Cellularity of bovine adipose tissues: Developmental changes from 15 to 65 percent mature weight. J. Lipid Res. 1981, 22, 452–457. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]







| Adipose Tissue Depot | mRNA Expression | Protein Expression |
|---|---|---|
| Epididymal Adipose Tissue | ||
| AQP9 | CC: NS | CC: NS |
| PT: NS | PT: NS | |
| RSV: NS | RSV: NS | |
| AQP7 | CC: NS | CC: NS |
| PT: NS | PT: NS | |
| RSV: NS | RSV: NS | |
| AQP3 | CC: ↑ 50% | CC: ↑ 100% |
| PT: NS | PT: NS | |
| RSV: ↑ 50% | RSV: ↑ 100% | |
| Subcutaneous Adipose Tissue | ||
| AQP9 | CC: ↑ 70% | |
| Not detected | PT: ↑ 200% | |
| RSV: NS | ||
| AQP7 | CC: NS | CC: NS |
| PT: ↓ 80% | PT: NS | |
| RSV: NS | RSV: NS | |
| AQP3 | CC: NS | CC: NS |
| PT: NS | PT: NS | |
| RSV: NS | RSV: NS | |
| Interscapular Brown Adipose Tissue | ||
| AQP9 | ||
| Not detected | Not detected | |
| AQP7 | CC: ↑ 80% | |
| PT: ↑ 70% | NS | |
| RSV: ↑ 100% | ||
| AQP3 | ||
| Not detected | Not detected | |
| Macronutrient | Standard Diet | Obesogenic Diet |
|---|---|---|
| Protein | 20% | 19% |
| Carbohydrates | 70% | 36% |
| Fat | 10% | 45% |
| 29% saturated | 36% saturated | |
| 37% monounsaturated | 47% monounsaturated | |
| 34% polyunsaturated | 17% polyunsaturated |
| Gene | Sense Primer Sequence | Antisense Primer Sequence | Annealing Temperature | Genbank Number |
|---|---|---|---|---|
| Aquaporin 3 | 5′-CCCCTTGTGATGCCTCTC-3′ | 5′-CCCTAGCTGGCAGAGTTC-3′ | EP 57.8 °C, SC 60 °C | NM_031703.1 |
| Aquaporin 7 | 5′-ATCCTTGTTTGCGTTCTTGG-3′ | 5′-GCGTGAATTAAGCCCAGGTA-3′ | EP 60 °C, SC 65.6 °C, IBAT 67 °C | NM_019157.2 |
| Aquaporin 9 | 5′-CTCAGTCCCAGGCTCTTCAC-3′ | 5′-ATGGCTCTGCCTTCATGTCT-3′ | EP 60 °C | NM_022960.2 |
| 18Sr RNA | 5′-GTGGGCCTGCGGCTTAAT-3′ | 5′-GCCAGAGTCTCGTTCGTTATC-3′ | 60 °C | M11188.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Zorita, S.; Trepiana, J.; Fernández-Quintela, A.; González, M.; Portillo, M.P. Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue. Int. J. Mol. Sci. 2018, 19, 2654. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092654
Gómez-Zorita S, Trepiana J, Fernández-Quintela A, González M, Portillo MP. Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue. International Journal of Molecular Sciences. 2018; 19(9):2654. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092654
Chicago/Turabian StyleGómez-Zorita, Saioa, Jenifer Trepiana, Alfredo Fernández-Quintela, Marcela González, and María P. Portillo. 2018. "Resveratrol and Pterostilbene, Two Analogue Phenolic Compounds, Affect Aquaglyceroporin Expression in a Different Manner in Adipose Tissue" International Journal of Molecular Sciences 19, no. 9: 2654. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092654