F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. FXIIIA Levels, Genotypes, and MACE
2.3. Survival Analysis and Predictive Model
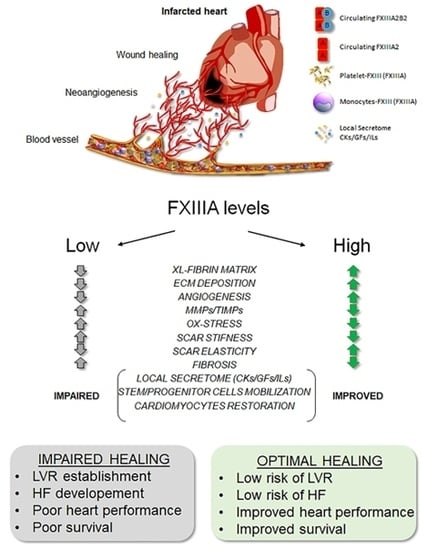
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Blood Samples and FXIIIA Level Measurements
4.3. Genotype Analysis
4.4. Follow-Up and Description of Endpoints
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gemmati, D.; Vigliano, M.; Burini, F.; Mari, R.; El Mohsein, H.H.; Parmeggiani, F.; Serino, M.L. Coagulation factor XIIIA (F13A1): Novel perspectives in treatment and pharmacogenetics. Curr. Pharm. Des. 2016, 22, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Zeri, G.; Orioli, E.; Mari, R.; Moratelli, S.; Vigliano, M.; Marchesini, J.; Grossi, M.E.; Pecoraro, A.; Cuneo, A.; et al. Factor XIII-A dynamics in acute myocardial infarction: A novel prognostic biomarker? Thromb. Haemost. 2015, 114, 123–132. [Google Scholar] [PubMed]
- Gemmati, D.; Federici, F.; Campo, G.; Tognazzo, S.; Serino, M.L.; De Mattei, M.; Valgimigli, M.; Malagutti, P.; Guardigli, G.; Ferraresi, P.; et al. Factor XIIIA-V34L and factor XIIIB-H95R gene variants: Effects on survival in myocardial infarction patients. Mol. Med. 2007, 13, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, R.P.; Bitar, A.; Owens, J.; Desta, Z.; Breall, J.A.; von der Lohe, E.; Sinha, A.; Vatta, M.; Nystrom, P.; Jin, Y.; et al. Factor XIII val34leu polymorphism and recurrent myocardial infarction in patients with coronary artery disease. J. Thromb. Thrombolysis 2014, 38, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guella, I.; Duga, S.; Ardissino, D.; Merlini, P.A.; Peyvandi, F.; Mannucci, P.M.; Asselta, R. Common variants in the haemostatic gene pathway contribute to risk of early-onset myocardial infarction in the italian population. Thromb. Haemost. 2011, 106, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Siegerink, B.; Algra, A.; Rosendaal, F.R. Genetic variants of coagulation factor XIII and the risk of myocardial infarction in young women. Br. J. Haematol. 2009, 146, 459–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereczky, Z.; Balogh, E.; Katona, E.; Czuriga, I.; Karpati, L.; Shemirani, A.H.; Edes, I.; Muszbek, L. Decreased factor XIII levels in factor XIII a subunit Leu34 homozygous patients with coronary artery disease. Thromb. Res. 2008, 121, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.; Katona, E.; Mezei, Z.A.; Kallai, J.; Gindele, R.; Edes, I.; Muszbek, L.; Papp, Z.; Bereczky, Z. Effect of factor XIII levels and polymorphisms on the risk of myocardial infarction in young patients. Mol. Cell. Biochem. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B. Fibrinogen and factor XIII at the intersection of coagulation, fibrinolysis and inflammation. Thromb. Haemost. 2014, 112, 649–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichinose, A. Factor XIII is a key molecule at the intersection of coagulation and fibrinolysis as well as inflammation and infection control. Int. J. Hematol. 2012, 95, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Inbal, A.; Dardik, R. Role of coagulation factor XIII (F XIII) in angiogenesis and tissue repair. Pathophysiol. Haemost. Thromb. 2006, 35, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Hu, K.; Frantz, S.; Jaffer, F.A.; Tung, C.H.; Hiller, K.H.; Voll, S.; Nordbeck, P.; Sosnovik, D.; Gattenlohner, S.; et al. Factor XIII deficiency causes cardiac rupture, impairs wound healing, and aggravates cardiac remodeling in mice with myocardial infarction. Circulation 2006, 113, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Aikawa, E.; Figueiredo, J.L.; Stangenberg, L.; van den Borne, S.W.; Blankesteijn, W.M.; Sosnovik, D.E.; Jaffer, F.A.; Tung, C.H.; Weissleder, R. Transglutaminase activity in acute infarcts predicts healing outcome and left ventricular remodelling: Implications for FXIII therapy and antithrombin use in myocardial infarction. Eur. Heart J. 2008, 29, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Dickneite, G.; Herwald, H.; Korte, W.; Allanore, Y.; Denton, C.P.; Matucci Cerinic, M. Coagulation factor XIII: A multifunctional transglutaminase with clinical potential in a range of conditions. Thromb. Haemost. 2015, 113, 686–697. [Google Scholar] [PubMed]
- Thomas, M.R.; Lip, G.Y. Novel risk markers and risk assessments for cardiovascular disease. Circ. Res. 2017, 120, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Stewart, A.D.; Miloszewski, K.J.; Losowsky, M.S.; Markham, A.F. Molecular basis of inherited factor XIII deficiency: Identification of multiple mutations provides insights into protein function. Br. J. Haematol. 1995, 91, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K.; Aikawa, E.; Stangenberg, L.; Wurdinger, T.; Figueiredo, J.L.; Libby, P.; Weissleder, R.; Pittet, M.J. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007, 204, 3037–3047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehberg, K.E.; Answini, G.; Wood, D.; Todd, J.; Julian, J.; Ogburn, N.; Allen, K.B. Intramyocardial injection of autologous platelet-rich plasma combined with transmyocardial revascularization. Cell Transplant. 2009, 18, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Velotta, J.; Brinton, T.J.; Wang, X.; Chang, S.; Palmer, O.; Sheikh, A.; Chung, J.; Yang, P.C.; Robbins, R.; et al. Revaten platelet-rich plasma improves cardiac function after myocardial injury. Cardiovasc. Revasc. Med. 2011, 12, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Zhou, X.; Zeng, S.; Ye, F.; Yun, J.L.; Huang, T.G.; Li, H.; Li, Y.M. Effects of intramyocardial injection of platelet-rich plasma on the healing process after myocardial infarction. Coron. Artery Dis. 2008, 19, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Anwar, R.; Gallivan, L.; Edmonds, S.D.; Markham, A.F. Genotype/phenotype correlations for coagulation factor XIII: Specific normal polymorphisms are associated with high or low factor XIII specific activity. Blood 1999, 93, 897–905. [Google Scholar] [PubMed]
- Attie-Castro, F.A.; Zago, M.A.; Lavinha, J.; Elion, J.; Rodriguez-Delfin, L.; Guerreiro, J.F.; Franco, R.F. Ethnic heterogeneity of the factor XIII val34leu polymorphism. Thromb. Haemost. 2000, 84, 601–603. [Google Scholar] [CrossRef] [PubMed]
- Kangsadalampai, S.; Board, P.G. The val34leu polymorphism in the a subunit of coagulation factor XIII contributes to the large normal range in activity and demonstrates that the activation peptide plays a role in catalytic activity. Blood 1998, 92, 2766–2770. [Google Scholar] [PubMed]
- Ariens, R.A.; Philippou, H.; Nagaswami, C.; Weisel, J.W.; Lane, D.A.; Grant, P.J. The factor XIII V34L polymorphism accelerates thrombin activation of factor XIII and affects cross-linked fibrin structure. Blood 2000, 96, 988–995. [Google Scholar] [PubMed]
- Wartiovaara, U.; Mikkola, H.; Szoke, G.; Haramura, G.; Karpati, L.; Balogh, I.; Lassila, R.; Muszbek, L.; Palotie, A. Effect of val34leu polymorphism on the activation of the coagulation factor XIII-A. Thromb. Haemost. 2000, 84, 595–600. [Google Scholar] [PubMed]
- Kohler, H.P.; Ariens, R.A.; Catto, A.J.; Carter, A.M.; Miller, G.J.; Cooper, J.A.; Mansfield, M.W.; Standeven, K.F.; Grant, P.J. Factor XIII A-subunit concentration predicts outcome in stroke subjects and vascular outcome in healthy, middle-aged men. Br. J. Haematol. 2002, 118, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, T.; Schroeder, V.; Windecker, S.; Meier, B.; Kohler, H.P. Venous and intracoronary factor XIII A-subunit antigen and activity levels are not associated with extent of coronary artery disease. J. Thromb. Haemost. 2003, 1, 861–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucher, N.; Schroeder, V.; Kohler, H.P. Role of blood coagulation factor XIII in patients with acute pulmonary embolism. Correlation of factor XIII antigen levels with pulmonary occlusion rate, fibrinogen, d-dimer, and clot firmness. Thromb. Haemost. 2003, 90, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, V.; Ortner, E.; Mono, M.L.; Galimanis, A.; Meier, N.; Findling, O.; Fischer, U.; Brekenfeld, C.; Arnold, M.; Mattle, H.P.; et al. Coagulation factor XIII activation peptide and subunit levels in patients with acute ischaemic stroke: A pilot study. Thromb. Res. 2010, 126, e122–e127. [Google Scholar] [CrossRef] [PubMed]
- Bereczky, Z.; Balogh, E.; Katona, E.; Czuriga, I.; Edes, I.; Muszbek, L. Elevated factor XIII level and the risk of myocardial infarction in women. Haematologica 2007, 92, 287–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Lange, M.; Andrew, T.; Snieder, H.; Ge, D.; Futers, T.S.; Standeven, K.; Spector, T.D.; Grant, P.J.; Ariens, R.A. Joint linkage and association of six single-nucleotide polymorphisms in the factor XIII-A subunit gene point to V34L as the main functional locus. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Martini, C.H.; Doggen, C.J.; Cavallini, C.; Rosendaal, F.R.; Mannucci, P.M. No effect of polymorphisms in prothrombotic genes on the risk of myocardial infarction in young adults without cardiovascular risk factors. J. Thromb. Haemost. 2005, 3, 177–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silvain, J.; Pena, A.; Vignalou, J.B.; Hulot, J.S.; Galier, S.; Cayla, G.; Bellemain-Appaix, A.; Barthelemy, O.; Beygui, F.; Bal-dit-Sollier, C.; et al. FXIII-A leu34 genetic variant in premature coronary artery disease: A genotype–phenotype case control study. Thromb. Haemost. 2011, 106, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Kohler, H.P.; Stickland, M.H.; Ossei-Gerning, N.; Carter, A.; Mikkola, H.; Grant, P.J. Association of a common polymorphism in the factor XIII gene with myocardial infarction. Thromb. Haemost. 1998, 79, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Aleksic, N.; Ahn, C.; Wang, Y.W.; Juneja, H.; Folsom, A.R.; Boerwinkle, E.; Wu, K.K. Factor XIIIA val34leu polymorphism does not predict risk of coronary heart disease: The atherosclerosis risk in communities (ARIC) study. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Song, G.G.; Kim, J.H.; Seo, Y.H.; Choi, S.J. Association of factor XIII val34leu polymorphism and coronary artery disease: A meta-analysis. Cardiol. J. 2017, 24, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Marin, F.; Gonzalez-Conejero, R.; Lee, K.W.; Corral, J.; Roldan, V.; Lopez, F.; Sogorb, F.; Caturla, J.; Lip, G.Y.; Vicente, V. A pharmacogenetic effect of factor XIII valine 34 leucine polymorphism on fibrinolytic therapy for acute myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 25–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldan, V.; Corral, J.; Marin, F.; Rivera, J.; Pineda, J.; Gonzalez-Conejero, R.; Sogorb, F.; Vicente, V. Role of factor XIII val34leu polymorphism in patients <45 years of age with acute myocardial infarction. Am. J. Cardiol. 2003, 91, 1242–1245. [Google Scholar] [PubMed]
- Wang, G.; Zou, Z.; Ji, X.; Ni, Q.; Ma, Z. Factor XIII-A val34leu polymorphism might beassociated with myocardial infarction risk: An updated meta-analysis. Int. J. Clin. Exp. Med. 2014, 7, 5547–5552. [Google Scholar] [PubMed]
- Guodong, J.; Beili, F.; Peng, C.; Oushan, T.; Jian, W.; Ji, M.; Yuping, S.; Geng, X. Coagulation factor XIII-A val34leu polymorphism and the risk of coronary artery disease and myocardial infarction in a chinese han population. Clin. Appl. Thromb. Hemost. 2011, 17, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Serino, M.L.; Ongaro, A.; Tognazzo, S.; Moratelli, S.; Resca, R.; Moretti, M.; Scapoli, G.L. A common mutation in the gene for coagulation factor XIII-A (val34leu): A risk factor for primary intracerebral hemorrhage is protective against atherothrombotic diseases. Am. J. Hematol. 2001, 67, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Szekely, E.G.; Czuriga-Kovacs, K.R.; Bereczky, Z.; Katona, E.; Mezei, Z.A.; Nagy, A.; Toth, N.K.; Berenyi, E.; Muszbek, L.; Csiba, L.; et al. Low factor XIII levels after intravenous thrombolysis predict short-term mortality in ischemic stroke patients. Sci. Rep. 2018, 8, 7662. [Google Scholar] [CrossRef] [PubMed]
- Bockeria, L.A.; Samsonova, N.N.; Yurlov, I.A.; Klimovich, L.G.; Kozar, E.F.; Olsen, E.H.; Zaets, S.B. Dynamics of factor XIII levels after open heart surgery for congenital heart defects: Do cyanotic and acyanotic patients differ? Pediatr. Cardiol. 2014, 35, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with st-segment elevation: The task force for the management of acute myocardial infarction in patients presenting with st-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent st-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Vanhoutte, D.; Heymans, S. Factor XIII: The cement of the heart after myocardial infarction? Eur. Heart J. 2008, 29, 427–428. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Weissleder, R.; Ertl, G. Does FXIII deficiency impair wound healing after myocardial infarction? PLoS ONE 2006, 1, e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarese, E.P.; Robinson, J.G.; Kowalewski, M.; Kolodziejczak, M.; Andreotti, F.; Bliden, K.; Tantry, U.; Kubica, J.; Raggi, P.; Gurbel, P.A. Association Between Baseline LDL-C Level and Total and Cardiovascular Mortality After LDL-C Lowering: A Systematic Review and Meta-analysis. JAMA 2018, 319, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; The Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Third universal definition of myocardial infarction. Eur. Heart J. 2012, 33, 2551–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gemmati, D.; Tognazzo, S.; Serino, M.L.; Fogato, L.; Carandina, S.; De Palma, M.; Izzo, M.; De Mattei, M.; Ongaro, A.; Scapoli, G.L.; et al. Factor XIII V34L polymorphism modulates the risk of chronic venous leg ulcer progression and extension. Wound Repair Regen. 2004, 12, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, P.; De Mattei, M.; Ongaro, A.; Fogato, L.; Carandina, S.; De Palma, M.; Tognazzo, S.; Scapoli, G.L.; Serino, M.L.; Caruso, A.; et al. Factor XIII contrasts the effects of metalloproteinases in human dermal fibroblast cultured cells. Vasc. Endovasc. Surg. 2004, 38, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Tognazzo, S.; Gemmati, D.; Palazzo, A.; Catozzi, L.; Carandina, S.; Legnaro, A.; Tacconi, G.; Scapoli, G.L.; Zamboni, P. Prognostic role of factor XIII gene variants in nonhealing venous leg ulcers. J. Vasc. Surg. 2006, 44, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Tognazzo, S.; Catozzi, L.; Federici, F.; De Palma, M.; Gianesini, S.; Scapoli, G.L.; De Mattei, M.; Liboni, A.; Zamboni, P. Influence of gene polymorphisms in ulcer healing process after superficial venous surgery. J. Vasc. Surg. 2006, 44, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.A.; Tcheng, J.E.; Bozkurt, B.; Chaitman, B.R.; Cutlip, D.E.; Farb, A.; Fonarow, G.C.; Jacobs, J.P.; Jaff, M.R.; Lichtman, J.H.; et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J. Am. Coll. Cardiol. 2015, 66, 403–469. [Google Scholar] [PubMed]





| Clinical and Demographic Characteristics | Whole Cohort (n = 333) | MACE+ (n = 115; 34.5%) | MACE− (n = 218; 65.5%) | p |
|---|---|---|---|---|
| Age, years (mean ± SD) | 69.2 ± 12.7 | 73.8 ± 11.34 | 65.01 ± 12.22 | <0.0001 |
| Male/female (n) (female %) | 235/98 (29.4) | 75/40 (34.8) | 160/58 (26.6) | n.s. |
| STEMI/NSTEMI (% stemi) | 240/93 (72.1) | 73/42 (63.5) | 167/51 (76.6) | 0.0145 |
| LVEF (%, mean ± SD) | 44.8 ± 11.0 | 40.9 ± 12.1 | 46.9 ± 10.0 | <0.0001 |
| Family History (%) | 33.9 | 23.9 | 39.2 | 0.0065 |
| Hypertension (%) | 66.4 | 76.1 | 61.2 | 0.0092 |
| Dyslipidaemia (%) | 36.0 | 34.5 | 36.8 | n.s. |
| Diabetes (%) | 23.2 | 37.2 | 15.8 | 0.0001 |
| Smoking Habit (%) | 54.7 | 46.9 | 58.8 | 0.046 |
| Previous MI (%) | 27.5 | 48.7 | 16.3 | 0.0001 |
| Fibrinogen (mg/dL) | 313.4 ± 107.6 | 355.1 ± 122.1 | 296.8 ± 91.4 | <0.0001 |
| CRP (mg/dL) | 2.6 ± 4.3 | 3.62 ± 5.16 | 2.05 ± 3.7 | 0.002 |
| TnI peak value (ng/mL, mean ± SD) | 4.92 ± 6.3 | 5.47 ± 7.3 | 3.9 ± 5.0 | 0.025 |
| CK-MB peak value (ng/mL, mean ± SD) | 137.9 ± 159 | 141.4 ± 205 | 136.1 ± 163 | n.s. |
| FXIIIA Genotype and Level | Whole Group (n = 333) | MACE+ (n = 115) | MACE− (n = 218) | p * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FXIIIA V34L n (%) | VV34 | VL34 | LL34 | VV34 | VL34 | LL34 | VV34 | VL34 | LL34 | |
| 207 (62.2) | 109 (32.7) | 17 (5.1) | 68 (59.1) | 40 (34.8) | 7 (6.1) | 139 (63.8) | 69 (31.7) | 10 (4.6) | n.s. | |
| 207 (62.2) | 126 (37.8) | 68 (59.1) | 47 (40.9) | 139 (63.8) | 79 (36.2) | n.s. | ||||
| FXIIIA-d0 (%, mean ± SD) | 99.4 ± 29.8 | 90.2 ± 29.4 | 103.8 ± 29.0 | <0.0001 | ||||||
| FXIIIA-d4 (%, mean ± SD) | 85.5 ± 29.8 | 74.5 ± 28.0 | 91.4 ± 28.4 | <0.0007 | ||||||
| p | <0.0001 | <0.0001 | <0.0001 | |||||||
| VV34 | L34-carriers | p | VV34 | L34-carriers | p | VV34 | L34-carriers | p | ||
| FXIIIA-d0 (%, mean ± SD) | 103.7 ± 28.6 | 92.4 ± 30.4 | 0.00092 | 93.5 ± 26.6 | 85.8 ± 32.6 | n.s. | 108.2 ± 28.3 | 96.1 ± 28.6 | 0.0029 | |
| FXIIIA-d4 (%, mean ± SD) | 90.1 ± 26.6 | 78.9 ± 26.7 | 0.0029 | 77.7 ± 26.5 | 69.8 ± 31.1 | n.s. | 96.8 ± 24.4 | 83.9 ± 22.6 | 0.0022 | |
| p | <0.00001 | 0.00096 | - | 0.0034 | 0.037 | - | 0.0027 | 0.0085 | - | |
| Clinical and Demographic Characteristics | Composite–MACE (All MACE) | MACE–Recurrence (Multiple MACE) * | ||
|---|---|---|---|---|
| p (Univariate) | p (Multivariate) | p (Univariate) | p (Multivariate) | |
| Age | <0.0001 | 0.040 | n.s. | -- |
| Sex (male) | 0.020 | n.s. | -- | -- |
| FXIIIAd0 | 0.0002 | n.s. | n.s. | -- |
| FXIIIAd4 | 0.0012 | n.s. | n.s. | -- |
| FXIIIAd0 < 76.8% | 0.00006 | 0.004 | n.s. | -- |
| FXIIIAd4 < 63.9% | 0.000001 | 0.0001 | n.s. | -- |
| FXIIIA V34L | n.s. | -- | 0.0195 | 0.0087 |
| Total cholesterol | <0.0001 | n.s. | n.s. | -- |
| LDL cholesterol | 0.0019 | n.s. | n.s. | -- |
| HDL cholesterol | n.s. | -- | n.s. | -- |
| Triglycerides | n.s. | -- | n.s. | -- |
| Serum creatinine—baseline | <0.0001 | n.s. | n.s. | -- |
| Serum creatinine—peak value | <0.0001 | n.s. | n.s. | -- |
| Fibrinogen | <0.0001 | n.s. | n.s. | -- |
| Previous AMI | <0.0001 | 0.0057 | n.s. | -- |
| Diabetes | <0.0001 | n.s. | n.s. | -- |
| TnI day-1 | 0.0382 | n.s. | n.s. | -- |
| CK-MB day-3 | 0.0148 | n.s. | 0.0438 | 0.0441 |
| CRP | 0.0046 | n.s. | n.s. | -- |
| Family history | 0.0060 | n.s. | n.s. | -- |
| Smoking habit | 0.0404 | n.s. | n.s. | -- |
| Hypertension | n.s. | -- | n.s. | -- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansani, L.; Marchesini, J.; Pestelli, G.; Luisi, G.A.; Scillitani, G.; Longo, G.; Milani, D.; Serino, M.L.; Tisato, V.; Gemmati, D. F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty. Int. J. Mol. Sci. 2018, 19, 2766. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092766
Ansani L, Marchesini J, Pestelli G, Luisi GA, Scillitani G, Longo G, Milani D, Serino ML, Tisato V, Gemmati D. F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty. International Journal of Molecular Sciences. 2018; 19(9):2766. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092766
Chicago/Turabian StyleAnsani, Lucia, Jlenia Marchesini, Gabriele Pestelli, Giovanni Andrea Luisi, Giulia Scillitani, Giovanna Longo, Daniela Milani, Maria Luisa Serino, Veronica Tisato, and Donato Gemmati. 2018. "F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty" International Journal of Molecular Sciences 19, no. 9: 2766. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms19092766