Curcumin and Heme Oxygenase: Neuroprotection and Beyond
Abstract
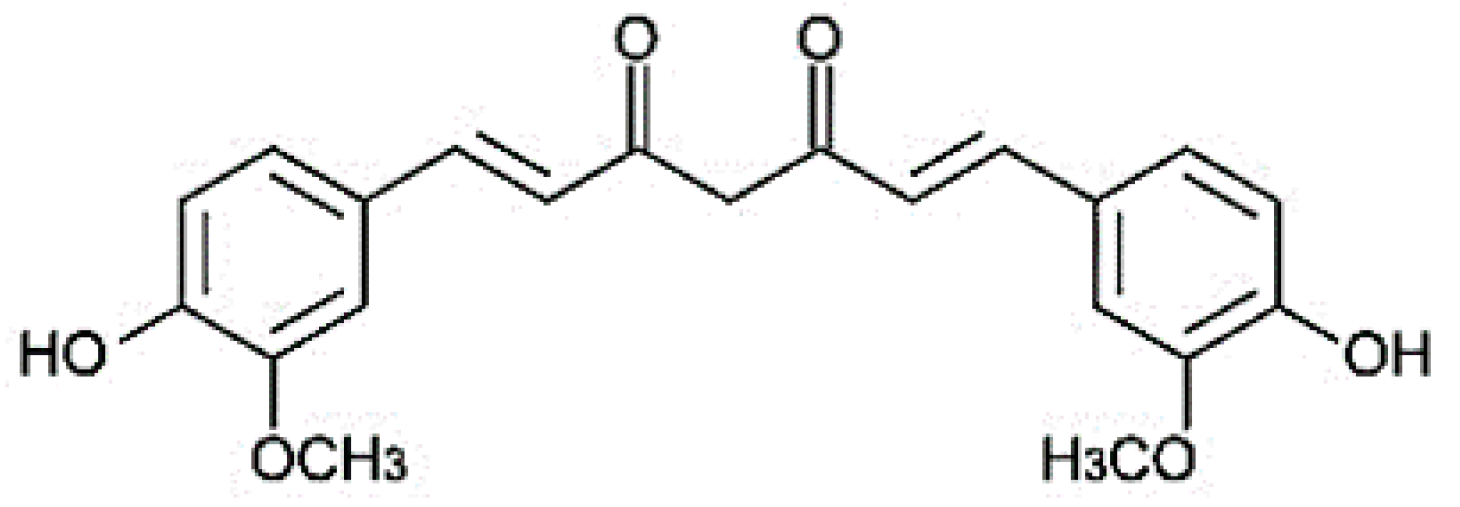
:1. Introduction
2. The Heme Oxygenase/Biliverdin Reductase Pathway
3. Curcumin, Neuroprotection, and the HO/BVR Pathway
4. Curcumin’s Safety Profile
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Den Haan, J.; Morrema, T.H.J.; Rozemuller, A.J.; Bouwman, F.H.; Hoozemans, J.J.M. Different Curcumin Forms Selectively Bind Fibrillar Amyloid Beta In Post Mortem Alzheimer’s Disease Brains: Implications for In-Vivo Diagnostics. Acta Neuropathol. Commun. 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Lestari, M.L.; Indrayanto, G. Profiles Drug Subst. Excip. Relat. Methodol. 2014, 39, 113–204. [Google Scholar]
- Jitoe-Masuda, A.; Fujimoto, A.; Masuda, T. Curcumin: From Chemistry to Chemistry-Based Functions. Curr. Pharm. Des. 2013, 19, 2084–2092. [Google Scholar]
- Mantzorou, M.; Pavlidou, E.; Vasios, G.; Tsagalioti, E.; Giaginis, C. Effects of Curcumin Consumption on Human Chronic Diseases: A Narrative Review of The Most Recent Clinical Data. Phytother. Res. 2018, 32, 957–975. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-Like Molecules: From Spice to Drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef]
- Calabrese, V.; Bates, T.E.; Mancuso, C.; Cornelius, C.; Ventimiglia, B.; Cambria, M.T.; Di Renzo, L.; de Lorenzo, A.; Dinkova-Kostova, A.T. Curcumin and The Cellular Stress Response in Free Radical-Related Diseases. Mol. Nutr. Food Res. 2008, 52, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Prado-Audelo, M.L.; Caballero-Floran, I.H.; Meza-Toledo, J.A.; Mendoza-Munoz, N.; Gonzalez-Torres, M.; Floran, B.; Cortes, H.; Leyva-Gomez, G. Formulations of Curcumin Nanoparticles for Brain Diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Siciliano, R.; Barone, E.; Preziosi, P. Natural Substances and Alzheimer’s Disease: From Preclinical Studies to Evidence Based Medicine. Biochim. Biophys. Acta 2012, 1822, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C. Bilirubin and Brain: A Pharmacological Approach. Neuropharmacology 2017, 118, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. The Heme Oxygenase System: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C. Heme Oxygenase and Its Products in The Nervous System. Antioxid. Redox Signal. 2004, 6, 878–887. [Google Scholar] [PubMed]
- Maines, M.D. The Heme Oxygenase System: Update 2005. Antioxid. Redox Signal. 2005, 7, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Santangelo, R.; Calabrese, V. The Heme Oxygenase/Biliverdin Reductase System: A Potential Drug Target in Alzheimers Disease. J. Biol. Regul. Homeost. Agents 2013, 27, 75–87. [Google Scholar] [PubMed]
- Mancuso, C.; Barone, E.; Guido, P.; Miceli, F.; Di Domenico, F.; Perluigi, M.; Santangelo, R.; Preziosi, P. Inhibition of Lipid Peroxidation and Protein Oxidation By Endogenous and Exogenous Antioxidants in Rat Brain Microsomes In Vitro. Neurosci. Lett. 2012, 518, 101–105. [Google Scholar] [CrossRef]
- Mancuso, C.; Tringali, G.; Grossman, A.; Preziosi, P.; Navarra, P. The Generation of Nitric Oxide and Carbon Monoxide Produces Opposite Effects on The Release of Immunoreactive Interleukin-1β From the Rat Hypothalamus In Vitro: Evidence for The Involvement of Different Signaling Pathways. Endocrinology 1998, 139, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, C.; Perluigi, M.; Cini, C.; de Marco, C.; Giuffrida Stella, A.M.; Calabrese, V. Heme Oxygenase and Cyclooxygenase in The Central Nervous System: A Functional Interplay. J. Neurosci. Res. 2006, 84, 1385–1391. [Google Scholar] [CrossRef]
- Suliman, H.B.; Piantadosi, C.A. Mitochondrial Biogenesis: Regulation by Endogenous Gases during Inflammation and Organ Stress. Curr. Pharm. Des. 2014, 20, 5653–5662. [Google Scholar] [CrossRef]
- Basuroy, S.; Tcheranova, D.; Bhattacharya, S.; Leffler, C.W.; Parfenova, H. Nox4 Nadph Oxidase-Derived Reactive Oxygen Species, Via Endogenous Carbon Monoxide, Promote Survival of Brain Endothelial Cells During Tnf-α-Induced Apoptosis. Am. J. Physiol. Cell Physiol. 2011, 300, C256–C265. [Google Scholar] [CrossRef]
- Santangelo, R.; Mancuso, C.; Marchetti, S.; di Stasio, E.; Pani, G.; Fadda, G. Bilirubin: An Endogenous Molecule with Antiviral Activity In Vitro. Front. Pharmacol. 2012, 3, 36. [Google Scholar] [CrossRef]
- Taille, C.; El-Benna, J.; Lanone, S.; Boczkowski, J.; Motterlini, R. Mitochondrial Respiratory Chain and Nad(P)H Oxidase Are Targets for The Antiproliferative Effect of Carbon Monoxide in Human Airway Smooth Muscle. J. Biol. Chem. 2005, 280, 25350–25360. [Google Scholar] [CrossRef]
- Jazwa, A.; Cuadrado, A. Targeting Heme Oxygenase-1 for Neuroprotection and Neuroinflammation in Neurodegenerative Diseases. Curr. Drug Targets 2010, 11, 1517–1531. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk Between Nrf2 And Nf-κb Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/Ho-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Sun, J.; Hoshino, H.; Takaku, K.; Nakajima, O.; Muto, A.; Suzuki, H.; Tashiro, S.; Takahashi, S.; Shibahara, S.; Alam, J.; et al. Hemoprotein Bach1 Regulates Enhancer Availability of Heme Oxygenase-1 Gene. Embo. J. 2002, 21, 5216–5224. [Google Scholar] [CrossRef]
- Kitamuro, T.; Takahashi, K.; Ogawa, K.; Udono-Fujimori, R.; Takeda, K.; Furuyama, K.; Nakayama, M.; Sun, J.; Fujita, H.; Hida, W.; et al. Bach1 Functions as A Hypoxia-Inducible Repressor for The Heme Oxygenase-1 Gene in Human Cells. J. Biol. Chem. 2003, 278, 9125–9133. [Google Scholar] [CrossRef]
- Motterlini, R.; Foresti, R.; Bassi, R.; Green, C.J. Curcumin, An Antioxidant and Anti-Inflammatory Agent, Induces Heme Oxygenase-1 And Protects Endothelial Cells Against Oxidative Stress. Free Radic. Biol. Med. 2000, 28, 1303–1312. [Google Scholar] [CrossRef]
- Scapagnini, G.; Foresti, R.; Calabrese, V.; Giuffrida Stella, A.M.; Green, C.J.; Motterlini, R. Caffeic Acid Phenethyl Ester and Curcumin: A Novel Class of Heme Oxygenase-1 Inducers. Mol. Pharmacol. 2002, 61, 554–561. [Google Scholar] [CrossRef] [Green Version]
- El-Bassossy, H.M.; El-Maraghy, N.N.; El-Fayoumi, H.M.; Watson, M.L. Haem Oxygenase-1 Induction Protects Against Tumour Necrosis Factor Alpha Impairment of Endothelial-Dependent Relaxation in Rat Isolated Pulmonary Artery. Br. J. Pharmacol. 2009, 158, 1527–1535. [Google Scholar] [CrossRef]
- Fang, X.D.; Yang, F.; Zhu, L.; Shen, Y.L.; Wang, L.L.; Chen, Y.Y. Curcumin Ameliorates High Glucose-Induced Acute Vascular Endothelial Dysfunction in Rat Thoracic Aorta. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1177–1182. [Google Scholar] [CrossRef]
- Hill-Kapturczak, N.; Thamilselvan, V.; Liu, F.; Nick, H.S.; Agarwal, A. Mechanism of Heme Oxygenase-1 Gene Induction by Curcumin In Human Renal Proximal Tubule Cells. Am. J. Physiol. Renal. Physiol. 2001, 281, F851–F859. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin Activates the Haem Oxygenase-1 Gene Via Regulation of Nrf2 and The Antioxidant-Responsive Element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Xu, Y.; Lian, Y.; Xie, N.; Wu, T.; Zhang, H.; Sun, L.; Zhang, R.; Wang, Z. Curcumin Improves Amyloid β-Peptide (1-42) Induced Spatial Memory Deficits Through Bdnf-Erk Signaling Pathway. PLoS ONE 2015, 10, E0131525. [Google Scholar] [CrossRef]
- Gaedeke, J.; Noble, N.A.; Border, W.A. Curcumin Blocks Fibrosis in Anti-Thy 1 Glomerulonephritis Through Up-Regulation of Heme Oxygenase 1. Kidney Int. 2005, 68, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Soetikno, V.; Sari, F.R.; Lakshmanan, A.P.; Arumugam, S.; Harima, M.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin Alleviates Oxidative Stress, Inflammation, And Renal Fibrosis in Remnant Kidney Through the Nrf2-Keap1 Pathway. Mol. Nutr. Food Res. 2013, 57, 1649–1659. [Google Scholar] [CrossRef]
- Mcnally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin Induces Heme Oxygenase 1 Through Generation of Reactive Oxygen Species, P38 Activation and Phosphatase Inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mcnally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin Induces Heme Oxygenase-1 In Hepatocytes and Is Protective in Simulated Cold Preservation and Warm Reperfusion Injury. Transplantation 2006, 81, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Li, K.; Rong, S.; Yao, P.; Hao, L.; Ying, C.; Zhang, X.; Nussler, A.; Liu, L. Curcumin Alleviates Ethanol-Induced Hepatocytes Oxidative Damage Involving Heme Oxygenase-1 Induction. J. Ethnopharmacol. 2010, 128, 549–553. [Google Scholar] [CrossRef]
- Rushworth, S.A.; Ogborne, R.M.; Charalambos, C.A.; O’connell, M.A. Role of Protein Kinase C Delta in Curcumin-Induced Antioxidant Response Element-Mediated Gene Expression in Human Monocytes. Biochem. Biophys. Res. Commun. 2006, 341, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Chu, L.C.; Hua, K.F.; Chao, L.K. Heme Oxygenase-1 Mediates the Anti-Inflammatory Effect of Curcumin Within Lps-Stimulated Human Monocytes. J. Cell Physiol. 2008, 215, 603–612. [Google Scholar] [CrossRef]
- Kim, K.M.; Pae, H.O.; Zhung, M.; Ha, H.Y.; Ha, Y.A.; Chai, K.Y.; Cheong, Y.K.; Kim, J.M.; Chung, H.T. Involvement of Anti-Inflammatory Heme Oxygenase-1 In the Inhibitory Effect of Curcumin on The Expression of Pro-Inflammatory Inducible Nitric Oxide Synthase in Raw264.7 Macrophages. Biomed. Pharmacother. 2008, 62, 630–636. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, T.; Lai, W.; Tan, Y.; Tian, D.; Guo, Z. Heme Oxygenase-1-Mediated Reactive Oxygen Species Reduction Is Involved in The Inhibitory Effect of Curcumin on Lipopolysaccharide-Induced Monocyte Chemoattractant Protein-1 Production in Raw264.7 Macrophages. Mol. Med. Rep. 2013, 7, 242–246. [Google Scholar] [CrossRef]
- Liu, L.; Shang, Y.; Li, M.; Han, X.; Wang, J.; Wang, J. Curcumin Ameliorates Asthmatic Airway Inflammation by Activating Nuclear Factor-E2-Related Factor 2/Haem Oxygenase (Ho)-1 Signalling Pathway. Clin. Exp. Pharmacol. Physiol. 2015, 42, 520–529. [Google Scholar] [CrossRef]
- Abuarqoub, H.; Green, C.J.; Foresti, R.; Motterlini, R. Curcumin Reduces Cold Storage-Induced Damage in Human Cardiac Myoblasts. Exp. Mol. Med. 2007, 39, 139–148. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, H.; Shi, Y. Upregulation of Heme Oxygenase-1 Expression by Curcumin Conferring Protection from Hydrogen Peroxide-Induced Apoptosis in H9c2 Cardiomyoblasts. Cell Biosci. 2017, 7, 20. [Google Scholar] [CrossRef]
- Pae, H.O.; Jeong, G.S.; Jeong, S.O.; Kim, H.S.; Kim, S.A.; Kim, Y.C.; Yoo, S.J.; Kim, H.D.; Chung, H.T. Roles of Heme Oxygenase-1 In Curcumin-Induced Growth Inhibition in Rat Smooth Muscle Cells. Exp. Mol. Med. 2007, 39, 267–277. [Google Scholar] [CrossRef]
- Olszanecki, R.; Gebska, A.; Korbut, R. The Role of Haem Oxygenase-1 in The Decrease of Endothelial Intercellular Adhesion Molecule-1 Expression by Curcumin. Basic Clin. Pharmacol. Toxicol. 2007, 101, 411–415. [Google Scholar] [CrossRef]
- Kanitkar, M.; Bhonde, R.R. Curcumin Treatment Enhances Islet Recovery by Induction of Heat Shock Response Proteins, Hsp70 And Heme Oxygenase-1, During Cryopreservation. Life Sci. 2008, 82, 182–189. [Google Scholar] [CrossRef]
- Abdel Aziz, M.T.; El-Asmar, M.F.; El Nadi, E.G.; Wassef, M.A.; Ahmed, H.H.; Rashed, L.A.; Obaia, E.M.; Sabry, D.; Hassouna, A.A.; Abdel Aziz, A.T. The Effect of Curcumin on Insulin Release in Rat-Isolated Pancreatic Islets. Angiology 2010, 61, 557–566. [Google Scholar] [CrossRef]
- Wei, S.M.; Yan, Z.Z.; Zhou, J. Curcumin Attenuates Ischemia-Reperfusion Injury in Rat Testis. Fertil. Steril. 2009, 91, 271–277. [Google Scholar] [CrossRef]
- Abd El-Fattah, A.A.; Fahim, A.T.; Sadik, N.A.H.; Ali, B.M. Resveratrol and Curcumin Ameliorate Di-(2-Ethylhexyl) Phthalate Induced Testicular Injury in Rats. Gen. Comp. Endocrinol. 2016, 225, 45–54. [Google Scholar] [CrossRef]
- Scharstuhl, A.; Mutsaers, H.A.; Pennings, S.W.; Szarek, W.A.; Russel, F.G.; Wagener, F.A. Curcumin-Induced Fibroblast Apoptosis And In Vitro Wound Contraction Are Regulated by Antioxidants and Heme Oxygenase: Implications for Scar Formation. J. Cell Mol. Med. 2009, 13, 712–725. [Google Scholar] [CrossRef]
- He, H.J.; Wang, G.Y.; Gao, Y.; Ling, W.H.; Yu, Z.W.; Jin, T.R. Curcumin Attenuates Nrf2 Signaling Defect, Oxidative Stress in Muscle and Glucose Intolerance in High Fat Diet-Fed Mice. World J. Diabetes 2012, 3, 94–104. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lee, Y.R.; Huang, C.C.; Li, Y.Z.; Chang, Y.S.; Yang, C.Y.; Wu, J.D.; Liu, Y.W. Curcumin-Induced Heme Oxygenase-1 Expression Plays A Negative Role for Its Anti-Cancer Effect in Bladder Cancers. Food Chem. Toxicol. 2012, 50, 3530–3536. [Google Scholar] [CrossRef]
- Lee, W.Y.; Chen, Y.C.; Shih, C.M.; Lin, C.M.; Cheng, C.H.; Chen, K.C.; Lin, C.W. The Induction of Heme Oxygenase-1 Suppresses Heat Shock Protein 90 And the Proliferation of Human Breast Cancer Cells Through Its Byproduct Carbon Monoxide. Toxicol. Appl. Pharmacol. 2014, 274, 55–62. [Google Scholar] [CrossRef]
- Chen, M.H.; Lee, M.Y.; Chuang, J.J.; Li, Y.Z.; Ning, S.T.; Chen, J.C.; Liu, Y.W. Curcumin Inhibits HCV Replication by Induction of Heme Oxygenase-1 And Suppression of Akt. Int. J. Mol. Med. 2012, 30, 1021–1028. [Google Scholar] [CrossRef]
- Han, S.; Xu, J.; Guo, X.; Huang, M. Curcumin Ameliorates Severe Influenza Pneumonia via Attenuating Lung Injury and Regulating Macrophage Cytokines Production. Clin. Exp. Pharmacol. Physiol. 2018, 45, 84–93. [Google Scholar] [CrossRef]
- Youn, G.S.; Kwon, D.J.; Ju, S.M.; Choi, S.Y.; Park, J. Curcumin Ameliorates TNF-α-Induced Icam-1 Expression and Subsequent Thp-1 Adhesiveness via The Induction of Heme Oxygenase-1 in The Hacat Cells. BMB Rep. 2013, 46, 410–415. [Google Scholar] [CrossRef]
- El-Bassossy, H.M.; Hassan, N.; Zakaria, M.N. Heme Oxygenase-1 Alleviates Vascular Complications Associated with Metabolic Syndrome: Effect on Endothelial Dependent Relaxation and No Production. Chem. Biol. Interact. 2014, 223, 109–115. [Google Scholar] [CrossRef]
- Scapagnini, G.; Colombrita, C.; Amadio, M.; D’agata, V.; Arcelli, E.; Sapienza, M.; Quattrone, A.; Calabrese, V. Curcumin Activates Defensive Genes and Protects Neurons Against Oxidative Stress. Antioxid. Redox Signal. 2006, 8, 395–403. [Google Scholar] [CrossRef]
- Shin, H.J.; Lee, J.Y.; Son, E.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; Choi, W.S.; Roh, G.S. Curcumin Attenuates the Kainic Acid-Induced Hippocampal Cell Death in the Mice. Neurosci. Lett. 2007, 416, 49–54. [Google Scholar] [CrossRef]
- Park, E.; Chun, H.S. Protective Effects of Curcumin on Manganese-Induced Bv-2 Microglial Cell Death. Biol. Pharm. Bull. 2017, 40, 1275–1281. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; Fan, H.; Liu, Y. Curcumin Upregulates Transcription Factor Nrf2, Ho-1 Expression and Protects Rat Brains Against Focal Ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, S.; Guzman-Beltran, S.; Medina-Campos, O.N.; Pedraza-Chaverri, J. Curcumin Pretreatment Induces Nrf2 And an Antioxidant Response and Prevents Hemin-Induced Toxicity in Primary Cultures of Cerebellar Granule Neurons Of Rats. Oxid. Med. Cell. Longev. 2013, 2013, 801418. [Google Scholar] [CrossRef]
- Cui, X.; Song, H.; Su, J. Curcumin Attenuates Hypoxic-Ischemic Brain Injury in Neonatal Rats Through Induction of Nuclear Factor Erythroid-2-Related Factor 2 And Heme Oxygenase-1. Exp. Ther. Med. 2017, 14, 1512–1518. [Google Scholar] [CrossRef]
- Wang, Y.F.; Gu, Y.T.; Qin, G.H.; Zhong, L.; Meng, Y.N. Curcumin Ameliorates the Permeability of The Blood-Brain Barrier During Hypoxia by Upregulating Heme Oxygenase-1 Expression in Brain Microvascular Endothelial Cells. J. Mol. Neurosci. 2013, 51, 344–351. [Google Scholar] [CrossRef]
- Eckert, G.P.; Schiborr, C.; Hagl, S.; Abdel-Kader, R.; Muller, W.E.; Rimbach, G.; Frank, J. Curcumin Prevents Mitochondrial Dysfunction in the Brain of the Senescence-Accelerated Mouse-Prone 8. Neurochem. Int. 2013, 62, 595–602. [Google Scholar] [CrossRef]
- Zheng, K.M.; Zhang, J.; Zhang, C.L.; Zhang, Y.W.; Chen, X.C. Curcumin Inhibits Appoptosin-Induced Apoptosis via Upregulating Heme Oxygenase-1 Expression in Sh-Sy5y Cells. Acta Pharmacol. Sin. 2015, 36, 544–552. [Google Scholar] [CrossRef]
- Cui, Q.; Li, X.; Zhu, H. Curcumin Ameliorates Dopaminergic Neuronal Oxidative Damage via Activation of The Akt/Nrf2 Pathway. Mol. Med. Rep. 2016, 13, 1381–1388. [Google Scholar] [CrossRef]
- Jin, M.; Park, S.Y.; Shen, Q.; Lai, Y.; Ou, X.; Mao, Z.; Lin, D.; Yu, Y.; Zhang, W. Anti-Neuroinflammatory Effect of Curcumin on Pam3csk4-Stimulated Microglial Cells. Int. J. Mol. Med. 2018, 41, 521–530. [Google Scholar] [CrossRef]
- Parada, E.; Buendia, I.; Navarro, E.; Avendano, C.; Egea, J.; Lopez, M.G. Microglial Ho-1 Induction by Curcumin Provides Antioxidant, Antineuroinflammatory, And Glioprotective Effects. Mol. Nutr. Food Res. 2015, 59, 1690–1700. [Google Scholar] [CrossRef]
- Woo, J.M.; Shin, D.Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin Protects Retinal Pigment Epithelial Cells Against Oxidative Stress Via Induction of Heme Oxygenase-1 Expression and Reduction of Reactive Oxygen. Mol. Vis. 2012, 18, 901–908. [Google Scholar]
- Fetoni, A.R.; Eramo, S.L.; Paciello, F.; Rolesi, R.; Podda, M.V.; Troiani, D.; Paludetti, G. Curcuma Longa (Curcumin) Decreases In Vivo Cisplatin-Induced Ototoxicity Through Heme Oxygenase-1 Induction. Otol. Neurotol. 2014, 35, E169–E177. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhang, X.; Li, Y. Protective Effects of Curcumin in Appswe Transfected Sh-Sy5y Cells. Neural. Regen Res. 2012, 7, 405–412. [Google Scholar]
- Mancuso, C.; Barone, E. Curcumin in Clinical Practice: Myth or Reality? Trends Pharmacol. Sci. 2009, 30, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose Escalation of a Curcuminoid Formulation. BMC Complement. Altern Med. 2006, 6, 10. [Google Scholar] [CrossRef]
- Garcea, G.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J.; Berry, D.P. Detection of Curcumin and Its Metabolites in Hepatic Tissue and Portal Blood of Patients Following Oral Administration. Br. J. Cancer 2004, 90, 1011–1015. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.; Cheung, S.K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients with Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of The Putative Chemopreventive Agent Curcumin by Cancer Patients: Assessment of Curcumin Levels in the Colorectum And Their Pharmacodynamic Consequences. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 120–125. [Google Scholar]
- Klickovic, U.; Doberer, D.; Gouya, G.; Aschauer, S.; Weisshaar, S.; Storka, A.; Bilban, M.; Wolzt, M. Human Pharmacokinetics of High Dose Oral Curcumin and Its Effect on Heme Oxygenase-1 Expression in Healthy Male Subjects. Biomed. Res. Int. 2014, 2014, 458592. [Google Scholar] [CrossRef]
- Jamwal, R. Bioavailable Curcumin Formulations: A Review of Pharmacokinetic Studies in Healthy Volunteers. J. Integr. Med. 2018, 16, 367–374. [Google Scholar] [CrossRef]
- Mancuso, C. Key Factors Which Concur to the Correct Therapeutic Evaluation of Herbal Products in Free Radical-Induced Diseases. Front. Pharmacol. 2015, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelova, A. Amphiphilic Nanocarrier Systems for Curcumin Delivery in Neurodegenerative Disorders. Medicines 2018, 5, 126. [Google Scholar] [CrossRef]
- Abdel Aziz, M.T.; El-Asmar, M.F.; El-Ibrashy, I.N.; Rezq, A.M.; Al-Malki, A.L.; Wassef, M.A.; Fouad, H.H.; Ahmed, H.H.; Taha, F.M.; Hassouna, A.A.; et al. Effect of Novel Water Soluble Curcumin Derivative On Experimental Type- 1 Diabetes Mellitus (Short Term Study). Diabetol. Metab. Syndr. 2012, 4, 30. [Google Scholar] [CrossRef]
- Abdel Aziz, M.T.; El Asmer, M.F.; Rezq, A.; Kumosani, T.A.; Mostafa, S.; Mostafa, T.; Atta, H.; Abdel Aziz Wassef, M.; Fouad, H.H.; Rashed, L.; et al. Novel Water-Soluble Curcumin Derivative Mediating Erectile Signaling. J. Sex. Med. 2010, 7, 2714–2722. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Gupta, R. Exosomes for The Enhanced Tissue Bioavailability and Efficacy of Curcumin. Aaps J. 2017, 19, 1691–1702. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-Primed Exosomes Potently Ameliorate Cognitive Function in Ad Mice by Inhibiting Hyperphosphorylation of The Tau Protein Through the Akt/Gsk-3beta Pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The Dark Side of Curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Baum, L.; Cheung, S.K.; Mok, V.C.; Lam, L.C.; Leung, V.P.; Hui, E.; Ng, C.C.; Chow, M.; Ho, P.C.; Lam, S.; et al. Curcumin Effects on Blood Lipid Profile in A 6-Month Human Study. Pharmacol. Res. 2007, 56, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, R.; Rahimi, R.; Farzaei, M.H. Pharmacokinetic Interactions of Curcuminoids With Conventional Drugs: A Review. J. Ethnopharmacol 2017, 209, 1–12. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; Mcardle, H.J.; Naska, A.; et al. Curcumin and Normal Functioning of Joints: Evaluation of A Health Claim Pursuant to Article 13(5) Of Regulation (Ec) No 1924/2006. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
| Preclinical Model | Curcumin (Concentration or Dose) | Effect(s) | Reference(s) |
|---|---|---|---|
| Endothelial cells | 2–30 µM | Enhancement of cellular resistance against oxidative damage. Alleviation of vasodilator dysfunction | [27,28,29,30] |
| Renal tubule cells | 1–50 µM | Cytoprotection. Inhibition of fibrosis. | [31,32,33] |
| Anti-Thy 1 glomerulonephritis rats Nephrectomized rats | 100 mg/kg i.p. 75 mg/kg per os | Reduction of renal fibrosis and proteinuria. Inhibition of lipid peroxidation, inflammation and renal fibrosis. Amelioration of renal function. | [34,35] |
| Hepatocytes | 1–50 µM | Cytoprotection against cold/rewarming- or ethanol-induced damages. | [36,37,38] |
| Monocytes | 1–20 µM | Activation of ARE-modulated genes via PKCδ. Inhibition of inflammation. | [39,40] |
| Macrophages | 0.5–50 µM | Inhibition of inflammation. | [41,42,43] |
| Cardiac myoblasts | 5–30 µM | Inhibition of apoptosis. Cytoprotection against cold-storage damage. | [44,45] |
| Smooth muscle cells | 1–20 µM | Inhibition of proliferation. | [46] |
| LPS-treated mice | 30 mg/kg i.p. | Prevention of pulmonary sequestration of neutrophils. | [47] |
| Pancreatic islets | 6–10 µM | Inhibition of islet damage during cryopreservation. Improvement of insulin secretion. | [48,49] |
| Rat testicular injury | 200 mg/kg i.v. 200 mg/kg per os for 30 days before and 45 days after injury. | Inhibition of lipid peroxidation and increase in testicular spermatogenesis. Reduced lipid peroxidation; improvement of serum testosterone level. | [50,51] |
| Fibroblasts | 5–25 µM | Induction of apoptosis and modulation of pathological scar formation. | [52] |
| High-fat-diet-fed mice | 50 mg/kg per os | Improvement in muscular oxidative stress and glucose tolerance. | [53] |
| Bladder cancer cells | 10 µM | Modulation of cancer cell proliferation. | [54] |
| Breast cancer cells | 5–20 µM | Inhibition of tumor invasion. | [55] |
| Hepatoma cells expressing HCV | 5–25 µM | Inhibition of HCV replication. | [56] |
| Lung cancer cells expressing influenza virus | 0.1–10 µM | Inhibition of virus-induced lung injury. | [57] |
| Keratinocytes | 1–30 µM | Anti-inflammatory activity. | [58] |
| Metabolic syndrome in rats | 5 mg/kg i.p. for 6 weeks | Prevention of hyperinsulinemia and amelioration of endothelial-dependent relaxation. | [59] |
| Formulation | AUC | Cmax | Tmax | T1/2 |
|---|---|---|---|---|
| Curcumin | ~312 ng/mL·h a | ~ 245 nM a | 0.5 h a | ~1.0 h a |
| Curcumin-PLGA | ~3224 ng/mL·h b | ~ 710 nM b | 2.0 h b | |
| Curcumin-TMC | ~12,760 ng/mL·h c | ~3.3 μM c | 2.0 h c | ~12 h c |
| Curcumin-SLN | ~42,000 ng/mL·h d | ~38 μM d | 0.5 h d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhillaj, E.; Tarozzi, A.; Pruccoli, L.; Cuomo, V.; Trabace, L.; Mancuso, C. Curcumin and Heme Oxygenase: Neuroprotection and Beyond. Int. J. Mol. Sci. 2019, 20, 2419. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20102419
Mhillaj E, Tarozzi A, Pruccoli L, Cuomo V, Trabace L, Mancuso C. Curcumin and Heme Oxygenase: Neuroprotection and Beyond. International Journal of Molecular Sciences. 2019; 20(10):2419. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20102419
Chicago/Turabian StyleMhillaj, Emanuela, Andrea Tarozzi, Letizia Pruccoli, Vincenzo Cuomo, Luigia Trabace, and Cesare Mancuso. 2019. "Curcumin and Heme Oxygenase: Neuroprotection and Beyond" International Journal of Molecular Sciences 20, no. 10: 2419. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20102419





