Impact of Periprosthetic Fibroblast-Like Cells on Osteoclastogenesis in Co-Culture with Peripheral Blood Mononuclear Cells Varies Depending on Culture System
Abstract
:1. Introduction
2. Results
2.1. TRAP and Hoechst Staining of Monolayer Cultures
2.2. Resorption Assay on Dentin
2.3. Quantitative Real Time PCR
2.3.1. Gene Expression in Monolayer Cultures
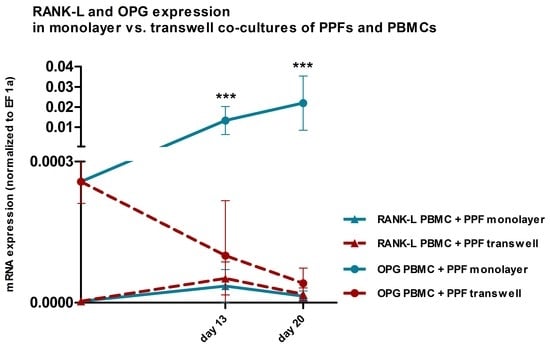
2.3.2. Comparison of Gene Expression in Monolayer and Transwell Co-Cultures
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Cell Culture
4.3. Isolation of PPFs
4.4. Isolation of PBMCs
4.5. Culture Conditions
4.6. Control Groups
4.7. Stimulation with MCSF and RANK-L
4.8. TRAP and Hoechst Staining of Monolayer Cultures
4.9. Resorption Assay on Dentin
4.10. RNA Isolation
4.11. Quantitative Real-Time PCR
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MCSF | Macrophage colony-stimulating factor |
| RANK-L | Receptor activator of NF-κB ligand |
| TNFα | Tumor necrosis factor alpha |
| OPG | Osteoprotegerin |
| PBMCs | Peripheral blood mononuclear cells |
| PPFs | Periprosthetic fibroblast-like cells |
| α-MEM | α-Minimal Essential Medium |
| DMEM | Dulbecco´s Minimal Essential Medium |
| PBS | Phosphate buffered saline |
| RT | Room temperature |
| TRAP | Tartrate resistant acid phosphatase |
| MNCs | Multinucleated cells |
| HKG | Housekeeping gene |
| EF 1 α | Elongation factor 1 α |
| CP | Crossing Point |
| ML | Monolayer |
| TW | Transwell |
References
- Souza, P.P.; Lerner, U.H. The role of cytokines in inflammatory bone loss. Immunol. Invest. 2013, 42, 555–622. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Gravallese, E. Bone erosion in rheumatoid arthritis: Mechanisms, diagnosis and treatment. Nat. Rev. Rheumatol. 2012, 8, 656–664. [Google Scholar] [CrossRef]
- Walsh, M.C.; Choi, Y. Biology of the RANKL-RANK-OPG System in Immunity, Bone, and Beyond. Front. Immunol. 2014, 5, 511. [Google Scholar] [CrossRef]
- Cobelli, N.; Scharf, B.; Crisi, G.M.; Hardin, J.; Santambrogio, L. Mediators of the inflammatory response to joint replacement devices. Nat. Rev. Rheumatol. 2011, 7, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Schoppet, M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004, 292, 490–495. [Google Scholar] [CrossRef]
- Quinn, J.M.; Horwood, N.J.; Elliott, J.; Gillespie, M.T.; Martin, T.J. Fibroblastic stromal cells express receptor activator of NF-kappa B ligand and support osteoclast differentiation. J. Bone Miner. Res. 2000, 15, 1459–1466. [Google Scholar] [CrossRef]
- Koreny, T.; Tunyogi-Csapo, M.; Gal, I.; Vermes, C.; Jacobs, J.J.; Glant, T.T. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum. 2006, 54, 3221–3232. [Google Scholar] [CrossRef]
- Sabokbar, A.; Itonaga, I.; Sun, S.G.; Kudo, O.; Athanasou, N.A. Arthroplasty membrane-derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. J. Orthop. Res. 2005, 23, 511–519. [Google Scholar] [CrossRef]
- Qian, Y.; Zeng, B.F.; Zhang, X.L.; Jiang, Y. Substance P stimulates production of interleukin 1beta and tumor necrosis factor alpha in fibroblasts from hip periprosthetic membrane. J. Arthroplasty 2008, 23, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Sadoghi, P.; Liebensteiner, M.; Agreiter, M.; Leithner, A.; Bohler, N.; Labek, G. Revision surgery after total joint arthroplasty: A complication-based analysis using worldwide arthroplasty registers. J. Arthroplasty 2013, 28, 1329–1332. [Google Scholar] [CrossRef]
- Saleh, K.J.; Celebrezze, M.; Kassim, R.; Dykes, D.C.; Gioe, T.J.; Callaghan, J.J.; Salvati, E.A. Functional outcome after revision hip arthroplasty: A metaanalysis. Clin. Orthop. Relat. Res. 2003, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.J.; Dykes, D.C.; Tweedie, R.L.; Mohamed, K.; Ravichandran, A.; Saleh, R.M.; Gioe, T.J.; Heck, D.A. Functional outcome after total knee arthroplasty revision: A meta-analysis. J. Arthroplasty 2002, 17, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Raska, M. Particle disease: Biologic mechanisms of periprosthetic osteolysis in total hip arthroplasty. Innate Immun. 2013, 19, 213–224. [Google Scholar] [CrossRef]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater 2013, 9, 8046–8058. [Google Scholar] [CrossRef] [Green Version]
- Jamsen, E.; Kouri, V.P.; Olkkonen, J.; Cor, A.; Goodman, S.B.; Konttinen, Y.T.; Pajarinen, J. Characterization of macrophage polarizing cytokines in the aseptic loosening of total hip replacements. J. Orthop. Res. 2014, 32, 1241–1246. [Google Scholar] [CrossRef] [PubMed]
- Mandelin, J.; Li, T.F.; Liljestrom, M.; Kroon, M.E.; Hanemaaijer, R.; Santavirta, S.; Konttinen, Y.T. Imbalance of RANKL/RANK/OPG system in interface tissue in loosening of total hip replacement. J. Bone Joint Surg. Br. 2003, 85, 1196–1201. [Google Scholar] [CrossRef]
- Crotti, T.N.; Smith, M.D.; Findlay, D.M.; Zreiqat, H.; Ahern, M.J.; Weedon, H.; Hatzinikolous, G.; Capone, M.; Holding, C.; Haynes, D.R. Factors regulating osteoclast formation in human tissues adjacent to peri-implant bone loss: Expression of receptor activator NFkappaB, RANK ligand and osteoprotegerin. Biomaterials 2004, 25, 565–573. [Google Scholar] [CrossRef]
- Hartmann, E.S.; Kohler, M.I.; Huber, F.; Redeker, J.I.; Schmitt, B.; Schmitt-Sody, M.; Summer, B.; Fottner, A.; Jansson, V.; Mayer-Wagner, S. Factors regulating bone remodeling processes in aseptic implant loosening. J. Orthop. Res. 2017, 35, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Goodman, S.B.; Huie, P.; Song, Y.; Schurman, D.; Maloney, W.; Woolson, S.; Sibley, R. Cellular profile and cytokine production at prosthetic interfaces. Study of tissues retrieved from revised hip and knee replacements. J. Bone Joint Surg. Br. 1998, 80, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Tomankova, T.; Kriegova, E.; Fillerova, R.; Luzna, P.; Ehrmann, J.; Gallo, J. Comparison of periprosthetic tissues in knee and hip joints: Differential expression of CCL3 and DC-STAMP in total knee and hip arthroplasty and similar cytokine profiles in primary knee and hip osteoarthritis. Osteoarthritis Cartilage 2014, 22, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Koulouvaris, P.; Ly, K.; Ivashkiv, L.B.; Bostrom, M.P.; Nestor, B.J.; Sculco, T.P.; Purdue, P.E. Expression profiling reveals alternative macrophage activation and impaired osteogenesis in periprosthetic osteolysis. J. Orthop. Res. 2008, 26, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Iizuka, H.; Juji, T.; Nakagawa, T.; Yamamoto, A.; Miyazaki, T.; Koshihara, Y.; Oda, H.; Nakamura, K.; Tanaka, S. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 259–269. [Google Scholar] [CrossRef]
- de Vries, T.J.; Schoenmaker, T.; Wattanaroonwong, N.; van den Hoonaard, M.; Nieuwenhuijse, A.; Beertsen, W.; Everts, V. Gingival fibroblasts are better at inhibiting osteoclast formation than periodontal ligament fibroblasts. J. Cell. Biochem. 2006, 98, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, T.J.; Suzuki, E.; Stanecki, C.; Shin, H.S.; Qui, H.; Adamopoulos, I.E. Rheumatoid and pyrophosphate arthritis synovial fibroblasts induce osteoclastogenesis independently of RANKL, TNF and IL-6. J. Autoimmun. 2012, 39, 369–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloemen, V.; Schoenmaker, T.; de Vries, T.J.; Everts, V. Direct cell-cell contact between periodontal ligament fibroblasts and osteoclast precursors synergistically increases the expression of genes related to osteoclastogenesis. J. Cell. Physiol. 2010, 222, 565–573. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Fernandes, M.H. Paracrine-mediated differentiation and activation of human haematopoietic osteoclast precursor cells by skin and gingival fibroblasts. Cell Prolif. 2011, 44, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Sabater, D.; Fernandez-Lopez, J.A.; Remesar, X.; Alemany, M. The use of Transwells improves the rates of differentiation and growth of cultured 3T3L1 cells. Anal. Bioanal. Chem. 2013, 405, 5605–5610. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Takayanagi, H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol. Metab. 2012, 23, 582–590. [Google Scholar] [CrossRef]
- Quinn, J.M.; Elliott, J.; Gillespie, M.T.; Martin, T.J. A combination of osteoclast differentiation factor and macrophage-colony stimulating factor is sufficient for both human and mouse osteoclast formation in vitro. Endocrinology 1998, 139, 4424–4427. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Grimaud, E.; Soubigou, L.; Couillaud, S.; Coipeau, P.; Moreau, A.; Passuti, N.; Gouin, F.; Redini, F.; Heymann, D. Receptor activator of nuclear factor kappaB ligand (RANKL)/osteoprotegerin (OPG) ratio is increased in severe osteolysis. Am. J. Pathol. 2003, 163, 2021–2031. [Google Scholar] [CrossRef]
- Granchi, D.; Pellacani, A.; Spina, M.; Cenni, E.; Savarino, L.M.; Baldini, N.; Giunti, A. Serum levels of osteoprotegerin and receptor activator of nuclear factor-kappaB ligand as markers of periprosthetic osteolysis. J. Bone Joint Surg. Am. 2006, 88, 1501–1509. [Google Scholar] [CrossRef]
- Mercatali, L.; Ricci, M.; Scarpi, E.; Serra, P.; Fabbri, F.; Ricci, R.; Liverani, C.; Zanoni, M.; Zoli, W.; Maltoni, R.; et al. RANK/RANK-L/OPG in patients with bone metastases treated with anticancer agents and zoledronic acid: A prospective study. Int. J. Mol. Sci. 2013, 14, 10683–10693. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.; Fissel, B.M.; Maeda, Y.; Yan, J.; Ge, X.; Gravallese, E.M.; Aliprantis, A.O.; Charles, J.F. RANK-Independent Osteoclast Formation and Bone Erosion in Inflammatory Arthritis. Arthritis Rheumatol 2016, 68, 2889–2900. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Sato, K.; Miyazaki, T.; Kitaura, H.; Kayama, H.; Miyoshi, F.; Araki, Y.; Akiyama, Y.; Takeda, K.; Mimura, T. Combination of tumor necrosis factor alpha and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheumatol 2014, 66, 121–129. [Google Scholar] [CrossRef]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef]
- Kim, N.; Kadono, Y.; Takami, M.; Lee, J.; Lee, S.H.; Okada, F.; Kim, J.H.; Kobayashi, T.; Odgren, P.R.; Nakano, H.; et al. Osteoclast differentiation independent of the TRANCE-RANK-TRAF6 axis. J. Exp. Med. 2005, 202, 589–595. [Google Scholar] [CrossRef]
- Sabokbar, A.; Kudo, O.; Athanasou, N.A. Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J. Orthop. Res. 2003, 21, 73–80. [Google Scholar] [CrossRef]
- Kook, S.H.; Son, Y.O.; Choe, Y.; Kim, J.H.; Jeon, Y.M.; Heo, J.S.; Kim, J.G.; Lee, J.C. Mechanical force augments the anti-osteoclastogenic potential of human gingival fibroblasts in vitro. J. Periodontal Res. 2009, 44, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Chiba, M.; Shimizu, Y.; Mitani, H. Periodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J. Bone Miner. Res. 2002, 17, 210–220. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Aihara, N.; Kojima, T.; Kasai, K. RANKL increase in compressed periodontal ligament cells from root resorption. J. Dent. Res. 2006, 85, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, E.S.; Köhler, M.I.; Beck, F.; Schlüssel, S.; Redeker, J.I.; Summer, B.; Fottner, A.; Jansson, V.; Mayer-Wagner, S. Fibroblast-like cells change gene expression of bone remodelling markers in 3D transwell cultures. in preparation.
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Itonaga, I.; Sabokbar, A.; Murray, D.W.; Athanasou, N.A. Effect of osteoprotegerin and osteoprotegerin ligand on osteoclast formation by arthroplasty membrane derived macrophages. Ann. Rheum. Dis. 2000, 59, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Athanasou, N.A. Cellular biology of bone-resorbing cells. J. Bone Joint Surg. Am. 1996, 78, 1096–1112. [Google Scholar] [CrossRef]
- Rivollier, A.; Mazzorana, M.; Tebib, J.; Piperno, M.; Aitsiselmi, T.; Rabourdin-Combe, C.; Jurdic, P.; Servet-Delprat, C. Immature dendritic cell transdifferentiation into osteoclasts: A novel pathway sustained by the rheumatoid arthritis microenvironment. Blood 2004, 104, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Hayman, A.R. Tartrate-resistant acid phosphatase (TRAP) and the osteoclast/immune cell dichotomy. Autoimmunity 2008, 41, 218–223. [Google Scholar] [CrossRef]
- Hayman, A.R.; Macary, P.; Lehner, P.J.; Cox, T.M. Tartrate-resistant acid phosphatase (Acp 5): Identification in diverse human tissues and dendritic cells. J. Histochem. Cytochem. 2001, 49, 675–684. [Google Scholar] [CrossRef]
- Hamalainen, H.K.; Tubman, J.C.; Vikman, S.; Kyrola, T.; Ylikoski, E.; Warrington, J.A.; Lahesmaa, R. Identification and validation of endogenous reference genes for expression profiling of T helper cell differentiation by quantitative real-time RT-PCR. Anal. Biochem. 2001, 299, 63–70. [Google Scholar] [CrossRef]





| Gene | Primer Sequences (5′–3′) | Annealing Temperature (AT) | Amplification (95 °C–AT–72 °C) | Amplicon Size (bp) |
|---|---|---|---|---|
| EF 1 α 1 | AGCGCCGGCTATGCCCCTG CTGAACCATCCAGGCCAAAT | 60 °C | 15 s–60 s–10 s | 59 |
| MCSF 2 | CCGAGGAGGTGTCGGAGTAC AATTTGGCACGAGGTCTCCAT | 60 °C | 10 s–10 s–15 s | 100 |
| RANK-L 2 | CATCCCATCTGGTTCCCATAA GCCCAACCCCGATCATG | 60 °C | 10 s–10 s–15 s | 60 |
| OPG 2 | CTGCGCGCTCGTGTTTC ACAGCTGATGAGAGGTTTCTTCGT | 60 °C | 30 s–60 s–15 s | 100 |
| TNFα 2 | CCCAGGGACCTCTCTCTAATC GCTTGAGGGTTTGCTACAACATG | 60 °C | 30 s–60 s–15 s | 103 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koehler, M.I.; Hartmann, E.S.; Schluessel, S.; Beck, F.; Redeker, J.I.; Schmitt, B.; Unger, M.; van Griensven, M.; Summer, B.; Fottner, A.; et al. Impact of Periprosthetic Fibroblast-Like Cells on Osteoclastogenesis in Co-Culture with Peripheral Blood Mononuclear Cells Varies Depending on Culture System. Int. J. Mol. Sci. 2019, 20, 2583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20102583
Koehler MI, Hartmann ES, Schluessel S, Beck F, Redeker JI, Schmitt B, Unger M, van Griensven M, Summer B, Fottner A, et al. Impact of Periprosthetic Fibroblast-Like Cells on Osteoclastogenesis in Co-Culture with Peripheral Blood Mononuclear Cells Varies Depending on Culture System. International Journal of Molecular Sciences. 2019; 20(10):2583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20102583
Chicago/Turabian StyleKoehler, Miriam I., Eliza S. Hartmann, Sabine Schluessel, Felicitas Beck, Julia I. Redeker, Baerbel Schmitt, Marina Unger, Martijn van Griensven, Burkhard Summer, Andreas Fottner, and et al. 2019. "Impact of Periprosthetic Fibroblast-Like Cells on Osteoclastogenesis in Co-Culture with Peripheral Blood Mononuclear Cells Varies Depending on Culture System" International Journal of Molecular Sciences 20, no. 10: 2583. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20102583