Single Crystal FLIM Characterization of Clofazimine Loaded in Silica-Based Mesoporous Materials and Zeolites
Abstract
:1. Introduction
2. Results and Discussion
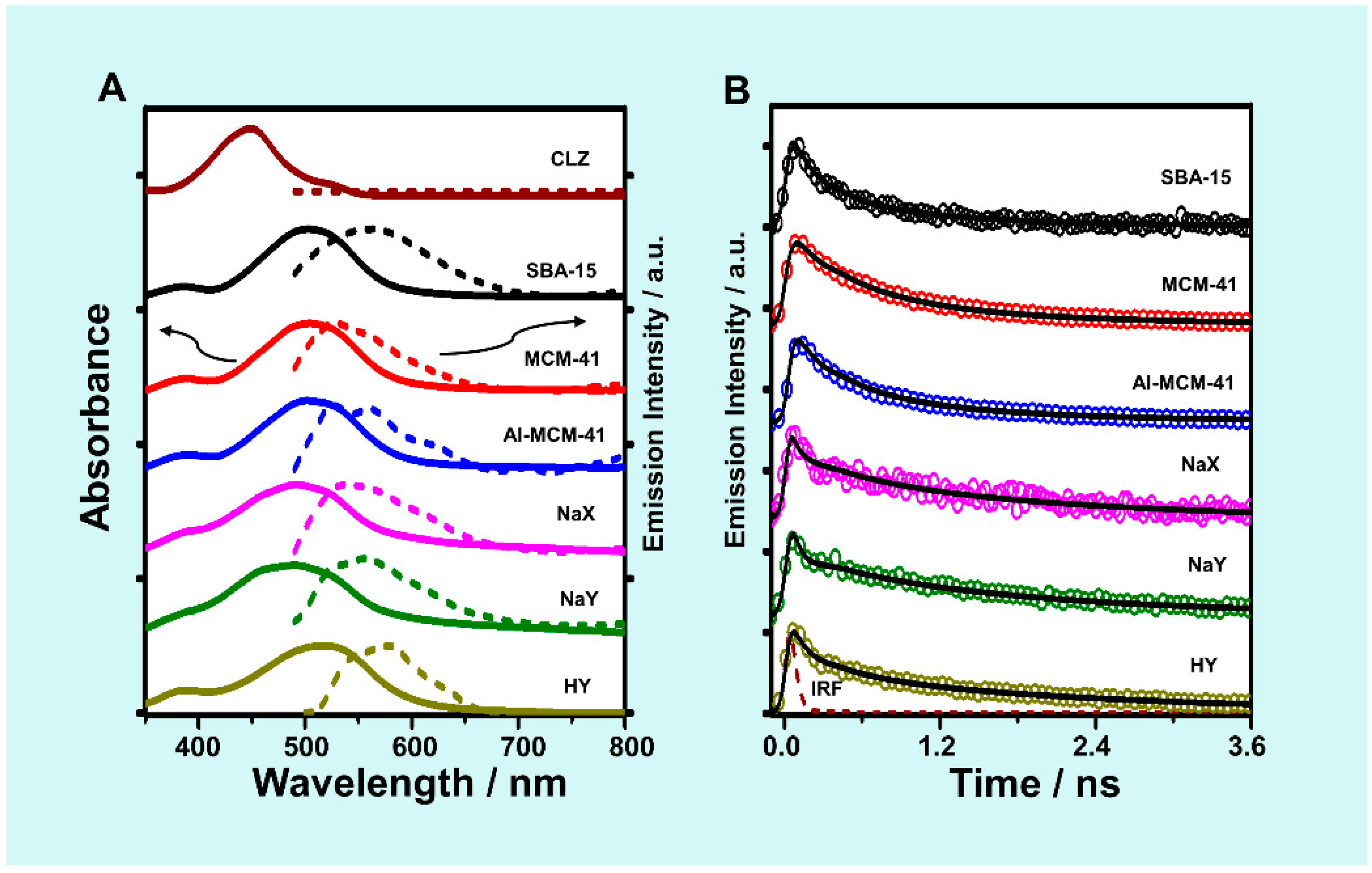
2.1. Steady-State Absorption and Diffuse Transmittance Spectra
2.2. CLZ Loading
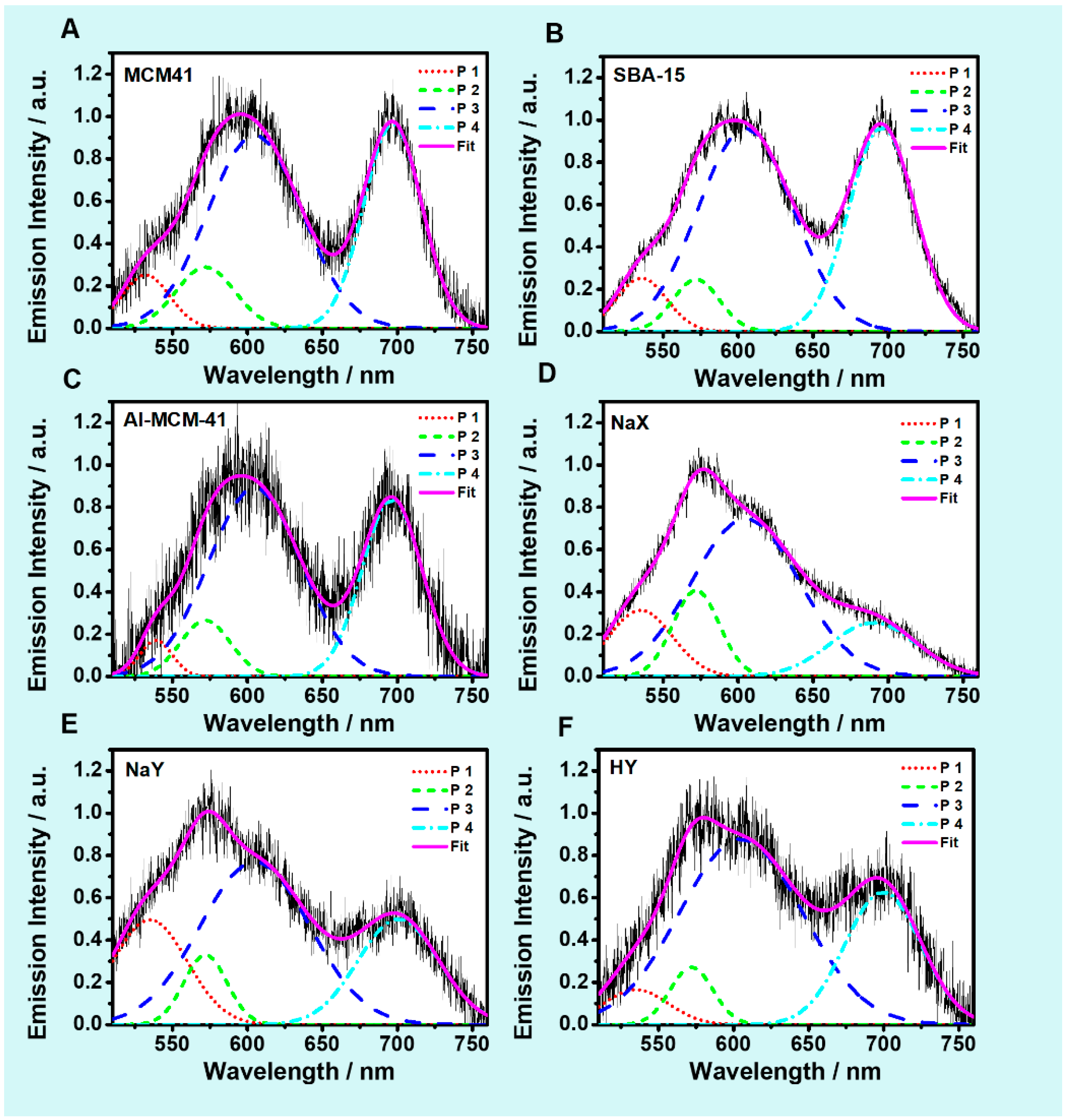
2.3. Steady-State Emission Spectra
2.4. TCSPC Decays in DCM Suspensions
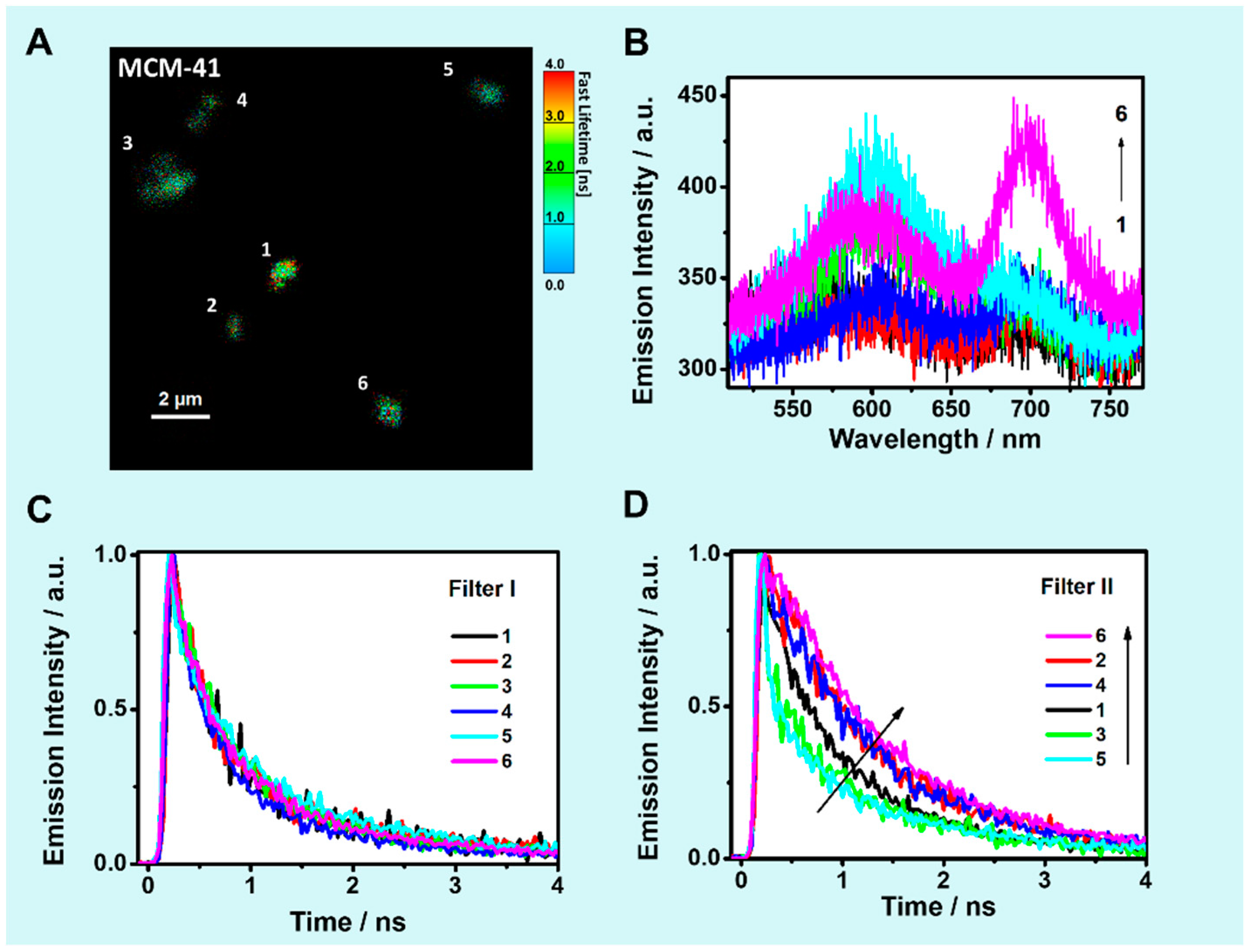
2.5. Solid State Scanning Confocal Fluorescence Microscopy
2.5.1. Single Particle Emission Spectra
2.5.2. TCSPC Decays of Solid Composites
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CLZ | Clofazimine |
| TCSPC | Time-correlated single-photon counting |
| FLIM | fluorescence lifetime imaging microscopy |
| MSPs | Mesoporous silica particles |
| DCM | Dichloromethane |
| DTS | Diffuse transmittance spectra |
| THF | Tetrahydrofuran |
| τi | Time constant |
| ai | Pre-exponential factor |
| ci | Fractional contributions |
| σ | Standard deviation |
| IRF | Instrumental response function |
References
- McKinney, C.W.; Pruden, A. Ultraviolet disinfection of antibiotic resistant bacteria and their antibiotic resistance genes in water and wastewater. Environ. Sci. Technol. 2012, 46, 13393–13400. [Google Scholar] [CrossRef] [PubMed]
- Barancheshme, F.; Munir, M. Strategies to combat antibiotic resistance in the wastewater treatment plants. Front. Microbiol. 2018, 8, 2603. [Google Scholar] [CrossRef] [PubMed]
- Lawe Davies, O.; Bennett, S. Who–List of Bacteria for Which New Antibiotics are Urgently Needed. Who Department of Communications. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 10 December 2018).
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Schott, M.-A.; Domurado, M.; Leclercq, L.; Barbaud, C.; Domurado, D. Solubilization of water-insoluble drugs due to random amphiphilic and degradable poly(dimethylmalic acid) derivatives. Biomacromolecules 2013, 14, 1936–1944. [Google Scholar] [CrossRef] [PubMed]
- Bannigan, P.; Zeglinski, J.; Lusi, M.; O’Brien, J.; Hudson, S.P. Investigation into the solid and solution properties of known and novel polymorphs of the antimicrobial molecule clofazimine. Cryst. Growth Des. 2016, 16, 7240–7250. [Google Scholar] [CrossRef]
- O’Connor, R.; O’Sullivan, J.F.; O’Kennedy, R. The pharmacology, metabolism, and chemistry of clofazimine. Drug Metab. Rev. 1995, 27, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Job, C.K.; Yoder, L.; Jacobson, R.R.; Hastings, R.C. Skin pigmentation from clofazimine therapy in leprosy patients: A reappraisal. J. Am. Acad. Dermatol. 1990, 23, 236–241. [Google Scholar] [CrossRef]
- Peters, K.; Leitzke, S.; Diederichs, J.E.; Borner, K.; Hahn, H.; Müller, R.H.; Ehlers, S. Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic efficacy in murine mycobacterium avium infection. J. Antimicrob. Chemother. 2000, 45, 77–83. [Google Scholar] [CrossRef]
- Salem, I.I.; Steffan, G.; Düzgünes, N. Efficacy of clofazimine–modified cyclodextrin against mycobacterium avium complex in human macrophages. Int. J. Pharm. 2003, 260, 105–114. [Google Scholar] [CrossRef]
- Adams, L.B.; Sinha, I.; Franzblau, S.G.; Krahenbuhl, J.L.; Mehta, R.T. Effective treatment of acute and chronic murine tuberculosis with liposome-encapsulated clofazimine. Antimicrob. Agents Chemother. 1999, 43, 1638–1643. [Google Scholar] [CrossRef]
- Stjern, L.; Voittonen, S.; Weldemichel, R.; Thuresson, S.; Agnes, M.; Benkovics, G.; Fenyvesi, É.; Malanga, M.; Yannakopoulou, K.; Feiler, A.; et al. Cyclodextrin-mesoporous silica particle composites for controlled antibiotic release. A proof of concept toward colon targeting. Int. J. Pharm. 2017, 531, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Pierro, P.; Palumbo, F.S.; Testa, F.; Pasqua, L.; Aiello, R. Drug delivery devices based on mesoporous silicate. Drug Deliv. 2004, 11, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Valetti, S.; Xia, X.; Costa-Gouveia, J.; Brodin, P.; Bernet-Camard, M.F.; Andersson, M.; Feiler, A. Clofazimine encapsulation in nanoporous silica particles for the oral treatment of antibiotic-resistant mycobacterium tuberculosis infections. Nanomedicine (Lond) 2017, 12, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Soleimani, H.A.; Mohammadpour, F.; Hadizadeh, F. Synthetic zeolites as controlled-release delivery systems for anti-inflammatory drugs. Chem. Biol. Drug Des. 2016, 87, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Aina, V.; Areán, C.; Balas, F.; Valentina, C.; Colilla, M.; Delgado, M.; Vallet-Regi, M. Studies on mcm-41 mesoporous silica for drug delivery: Effect of particle morphology and amine functionalization. Chem. Eng. J. 2008, 137, 30–37. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Rimoli, M.G.; Rabaioli, M.R.; Melisi, D.; Curcio, A.; Mondello, S.; Mirabelli, R.; Abignente, E. Synthetic zeolites as a new tool for drug delivery. J. Biomed. Mater. Res. Part A 2008, 87, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z.; Zahraee, Z. Controlled release of metformin from chitosan–based nanocomposite films containing mesoporous mcm-41 nanoparticles as novel drug delivery systems. J. Colloid Interface Sci. 2017, 501, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A new property of mcm-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Muñoz, B.; Rámila, A.; Pérez-Pariente, J.; Díaz, I.; Vallet-Regí, M. Mcm-41 organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Alarcos, N.; Cohen, B.; Ziółek, M.; Douhal, A. Photochemistry and photophysics in silica-based materials: Ultrafast and single molecule spectroscopy observation. Chem. Rev. 2017, 117, 13639–13720. [Google Scholar] [CrossRef] [PubMed]
- Angiolini, L.; Valetti, S.; Cohen, B.; Feiler, A.; Douhal, A. Fluorescence imaging of antibiotic clofazimine encapsulated within mesoporous silica particle carriers: Relevance to drug delivery and the effect on its release kinetics. Phys. Chem. Chem. Phys. 2018, 20, 11899–11911. [Google Scholar] [CrossRef] [PubMed]
- Valetti, S.; Wankar, J.; Ericson, M.B.; Feiler, A.; Manet, I. Mesoporous silica particles as a lipophilic drug vehicle investigated by fluorescence lifetime imaging. J. Mater. Chem. B 2017, 5, 3201–3211. [Google Scholar] [CrossRef]
- Giri, S.; Trewyn, B.G.; Lin, V.S. Mesoporous silica nanomaterial-based biotechnological and biomedical delivery systems. Nanomedicine (Lond) 2007, 2, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Galán, M.; Navascués, N.; Téllez, C.; Marquina, C.; Ibarra, M.R.; Santamaría, J. Development of magnetic nanostructured silica-based materials as potential vectors for drug-delivery applications. Chem. Mater. 2006, 18, 1911–1919. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A mesoporous silica nanosphere-based carrier system with chemically removable cds nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Wankar, J.; Bonvicini, F.; Benkovics, G.; Marassi, V.; Malanga, M.; Fenyvesi, E.; Gentilomi, G.A.; Reschiglian, P.; Roda, B.; Manet, I. Widening the therapeutic perspectives of clofazimine by its loading in sulfobutylether β-cyclodextrin nanocarriers: Nanomolar ic50 values against mdr s. Epidermidis. Mol. Pharm. 2018, 15, 3823–3836. [Google Scholar] [CrossRef]
- Yano, T.; Kassovska-Bratinova, S.; Teh, J.-S.; Winkler, J.; Sullivan, K.; Isaacs, A.; Schechter, N.M.; Rubin, H. Reduction of clofazimine by mycobacterial type 2 nadh:Quinone oxidoreductase: A pathway for the generation of bactericidal levels of reactive oxygen species. J. Biol. Chem. 2010, 286, 10276–10287. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, A.V. Non-specific and specific interactions of molecules of different electronic structures with solid surfaces. Discuss. Faraday Soc. 1965, 40, 205–218. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Clofazimine mesylate: A high solubility stable salt. Cryst. Growth Des. 2012, 12, 6250–6259. [Google Scholar] [CrossRef]
- Nie, H.C.; Su, Y.C.; Zhang, M.T.; Song, Y.; Leone, A.; Taylor, L.S.; Marsac, P.J.; Li, T.L.; Byrn, S.R. Solid-state spectroscopic investigation of molecular interactions between clofazimine and hypromellose phthalate in amorphous solid dispersions. Mol. Pharm. 2016, 13, 3964–3975. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Mo, H.; Zhang, M.; Song, Y.; Fang, K.; Taylor, L.S.; Li, T.; Byrn, S.R. Investigating the interaction pattern and structural elements of a drug–polymer complex at the molecular level. Mol. Pharm. 2015, 12, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Wang, S.; Organero, J.A.; Teruel, L.; Garcia, H.; Douhal, A. Femtosecond dynamics within nanotubes and nanocavities of mesoporous and zeolite materials. J. Phys. Chem. C 2009, 113, 11614–11622. [Google Scholar] [CrossRef]
- Karge, H.G.; Raskó, J. Hydrogen sulfide adsorption on faujasite-type zeolites with systematically varied si-al ratios. J. Colloid Interface Sci. 1978, 64, 522–532. [Google Scholar] [CrossRef]
- Gil, M.; Ziółek, M.; Organero, J.A.; Douhal, A. Confined fast and ultrafast dynamics of a photochromic proton-transfer dye within a zeolite nanocage. J. Phys. Chem. C 2010, 114, 9554–9562. [Google Scholar] [CrossRef]
- Vajtai, R. Springer Handbook of Nanomaterials; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Martin, C.; Cohen, B.; Navarro, M.T.; Corma, A.; Douhal, A. Unraveling the ultrafast behavior of nile red interacting with aluminum and titanium co-doped mcm41 materials. Phys. Chem. Chem. Phys. 2016, 18, 2152–2163. [Google Scholar] [CrossRef] [PubMed]
- Leal Marchena, C.; Lerici, L.; Renzini, S.; Pierella, L.; Pizzio, L. Synthesis and characterization of a novel tungstosilicic acid immobilized on zeolites catalyst for the photodegradation of methyl orange. Appl. Catal. B 2016, 188, 23–30. [Google Scholar] [CrossRef]
- Gil, M.; Organero, J.A.; Peris, E.; García, H.; Douhal, A. Confinement effect of nanocages and nanotubes of mesoporous materials on the keto forms photodynamics of sudan i. Chem. Phys. Lett. 2009, 474, 325–330. [Google Scholar] [CrossRef]
- Martin, C.; Piatkowski, P.; Cohen, B.; Gil, M.; Navarro, M.T.; Coma, A.; Douhal, A. Ultrafast dynamics of nile red interacting with metal doped mesoporous materials. J. Phys. Chem. C 2015, 119, 13283–13296. [Google Scholar] [CrossRef]
- Alarcos, N.; Organero, J.A.; Sánchez, F.; Douhal, A. Exploring the photobehavior of nanocaged monomers and h- and j-aggregates of a proton-transfer dye within nax and nay zeolites. J. Phys. Chem. C 2014, 118, 8217–8226. [Google Scholar] [CrossRef]
- Su, F.; Zhao, X.S.; Lv, L.; Zhou, Z. Synthesis and characterization of microporous carbons templated by ammonium-form zeolite y. Carbon 2004, 42, 2821–2831. [Google Scholar] [CrossRef]
- Grünberg, B.; Emmler, T.; Gedat, E.; Shenderovich, I.; Findenegg, G.H.; Limbach, H.H.; Buntkowsky, G. Hydrogen bonding of water confined in mesoporous silica mcm-41 and sba-15 studied by 1h solid-state nmr. Chem.–A Eur. J. 2004, 10, 5689–5696. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-I.; Rajesh, K.; Stanton, J.F.; Baiz, C.R. Quantifying hydrogen-bond populations in dimethyl sulfoxide/water mixtures. Angew. Chem. Int. Ed. 2017, 56, 11375–11379. [Google Scholar] [CrossRef] [PubMed]
- Keswani, R.K.; Baik, J.; Yeomans, L.; Hitzman, C.; Johnson, A.M.; Pawate, A.S.; Kenis, P.J.A.; Rodriguez-Homedo, N.; Stringer, K.A.; Rosania, G.R. Chemical analysis of drug biocrystals: A role for counterion transport pathways in intracellular drug disposition. Mol. Pharm. 2015, 12, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Rychlewska, U.; Broom, M.B.H.; Eggleston, D.S.; Hodgson, D.J. Antileprosy dihydrophenazines. Structural characterization of two crystal forms of clofazimine and of isoclofazimine, b.3857. J. Am. Chem. Soc. 1985, 107, 4768–4772. [Google Scholar] [CrossRef]
- Mammen, M.; Simanek, E.E.; Whitesides, G.M. Predicting the relative stabilities of multiparticle hydrogen-bonded aggregates based on the number of hydrogen bonds and the number of particles and measuring these stabilities with titrations using dimethyl sulfoxide. J. Am. Chem. Soc. 1996, 118, 12614–12623. [Google Scholar] [CrossRef]
- Lodi, A.; Ponterini, G. J-aggregation of an anionic oxacarbocyanine in electrostatically self-assembled multilayers. Thin Solid Film. 2006, 496, 585–594. [Google Scholar] [CrossRef]
- Busby, M.; Blum, C.; Tibben, M.; Fibikar, S.; Calzaferri, G.; Subramaniam, V.; De Cola, L. Time, space, and spectrally resolved studies on j-aggregate interactions in zeolite l nanochannels. J. Am. Chem. Soc. 2008, 130, 10970–10976. [Google Scholar] [CrossRef]
- Alarcos, N.; Sánchez, F.; Douhal, A. Spectroscopy and relaxation dynamics of salicylideneaniline derivative aggregates encapsulated in mcm41 and sba15 pores. Microporous Mesoporous Mater. 2016, 226, 34–43. [Google Scholar] [CrossRef]
- Eisfeld, A.; Briggs, J.S. The j- and h-bands of organic dye aggregates. Chem. Phys. 2006, 324, 376–384. [Google Scholar] [CrossRef]




| Host Material | CLZ Loading (%) | CLZ Content Capacity (% w/w) | Pore Size (Å) | Pore Volume (cm3 g−1) | Ratio Si/Al |
|---|---|---|---|---|---|
| SBA-15 | >99% | 4 | 60 | 0.7–0.9 a | 0 |
| MCM-41 | 81 | 3.1 | 25 | 0.98 b | 0 |
| Al-MCM-41 | 84 | 3.3 | 25 | 1 b | 30 |
| NaX | 40 | 1.6 | 13 | 0.29 c | 1.24 |
| NaY | 51 | 1.2 | 13 | 0.34 c | 2.45 |
| HY | 46 | 1.8 | 7.4 | 0.33 d | 2.47 |
| Host Material | λ obs (nm) | τ1 (ns) 1 | a1 (%) | τ2 (ns) 1 | a2 (%) |
|---|---|---|---|---|---|
| SBA-15 | 525 | 0.1 | 89 | 1.4 | 11 |
| 550 | 0.1 | 93 | 1.6 | 7 | |
| 575 | 0.2 | 93 | 1.8 | 7 | |
| 600 | 0.2 | 91 | 1.5 | 9 | |
| MCM-41 | 550 | 0.2 | 45 | 0.9 | 55 |
| 600 | 0.2 | 44 | 0.9 | 56 | |
| 650 | 0.2 | 46 | 0.9 | 54 | |
| Al-MCM-41 | 550 | 0.2 | 60 | 1.1 | 40 |
| 600 | 0.3 | 57 | 1.1 | 43 | |
| 650 | 0.2 | 52 | 1.0 | 48 | |
| NaX | 525 | 0.1 | 94 | 1.8 | 6 |
| 550 | 0.1 | 63 | 1.8 | 37 | |
| 575 | 0.1 | 64 | 1.7 | 36 | |
| 600 | 0.1 | 60 | 1.7 | 40 | |
| NaY | 525 | 0.1 | 97 | 1.7 | 3 |
| 550 | 0.1 | 85 | 2.0 | 15 | |
| 575 | 0.1 | 88 | 1.9 | 12 | |
| 600 | 0.1 | 86 | 2.0 | 14 | |
| HY | 525 | 0.4 | 50 | 2.2 | 50 |
| 550 | 0.3 | 49 | 2.2 | 51 | |
| 575 | 0.3 | 50 | 2.2 | 50 | |
| 600 | 0.2 | 55 | 2.2 | 45 |
| Host Material | τ11 (ns) | a1 (%) | c1 (%) | τ22 (ns) | σ2 | a2 (%) | c2 (%) | τ32 (ns) | σ3 | a3 (%) | c3 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBA-15 | 0.1 | 45 | 9 | 0.6 | 0.08 | 49 | 58 | 2.6 | 0.32 | 6 | 33 |
| MCM-41 | 0.1 | 45 | 7 | 0.7 | 0.09 | 46 | 50 | 3.2 | 0.57 | 8 | 43 |
| Al-MCM-41 | 0.1 | 57 | 12 | 0.7 | 0.12 | 36 | 46 | 3.4 | 0.40 | 7 | 42 |
| NaX | 0.1 | 63 | 16 | 0.6 | 0.07 | 33 | 52 | 3.1 | 0.37 | 4 | 32 |
| NaY | 0.1 | 81 | 36 | 0.4 | 0.07 | 17 | 36 | 3.3 | 0.74 | 2 | 28 |
| HY | 0.1 | 87 | 52 | 0.5 | 0.09 | 12 | 32 | 3.6 | 1.03 | 1 | 16 |
| Host Material | τ11 (ns) | a1 (%) | c1 (%) | τ22 (ns) | σ2 | a2 (%) | c2 (%) | τ32 (ns) | σ3 | a3 (%) | c3 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBA-15 | 0.1 | 40 | 8 | 0.9 | 0.12 | 45 | 53 | 1.8 | 0.15 | 15 | 39 |
| MCM-41 | 0.1 | 43 | 6 | 0.9 | 0.19 | 44 | 52 | 2.2 | 0.43 | 13 | 42 |
| Al-MCM-41 | 0.1 | 41 | 9 | 0.8 | 0.17 | 51 | 65 | 2.0 | 0.67 | 8 | 26 |
| NaX | 0.1 | 49 | 15 | 0.5 | 0.04 | 46 | 64 | 1.6 | 0.22 | 5 | 21 |
| NaY | 0.1 | 47 | 16 | 0.4 | 0.03 | 50 | 72 | 1.3 | 0.38 | 3 | 12 |
| HY | 0.1 | 46 | 18 | 0.4 | 0.02 | 52 | 76 | 1.3 | 0.30 | 2 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angiolini, L.; Cohen, B.; Douhal, A. Single Crystal FLIM Characterization of Clofazimine Loaded in Silica-Based Mesoporous Materials and Zeolites. Int. J. Mol. Sci. 2019, 20, 2859. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122859
Angiolini L, Cohen B, Douhal A. Single Crystal FLIM Characterization of Clofazimine Loaded in Silica-Based Mesoporous Materials and Zeolites. International Journal of Molecular Sciences. 2019; 20(12):2859. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122859
Chicago/Turabian StyleAngiolini, Lorenzo, Boiko Cohen, and Abderrazzak Douhal. 2019. "Single Crystal FLIM Characterization of Clofazimine Loaded in Silica-Based Mesoporous Materials and Zeolites" International Journal of Molecular Sciences 20, no. 12: 2859. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122859