Y Chromosome, Hypertension and Cardiovascular Disease: Is Inflammation the Answer?
Abstract
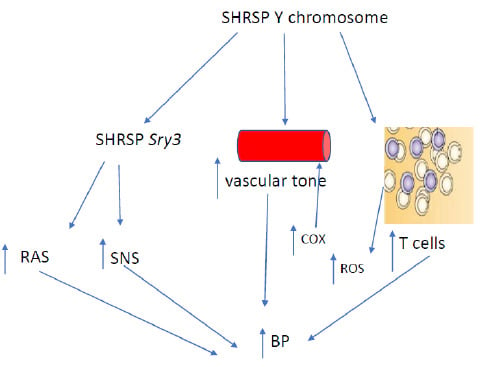
:1. Introduction
2. Y Chromosome and Hypertension: Studies in Rodents
3. Y Chromosome, Cardiovascular Disease and Hypertension: Human Studies
4. Y Chromosome and Vascular Function
5. Y Chromosome and Inflammation
6. Y Chromosome, T Cells and Hypertension
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skaletsky, H.; Kuroda-Kawaguchi, T.; Minx, P.J.; Cordum, H.S.; Hillier, L.; Brown, L.G.; Repping, S.; Pyntikova, T.; Ali, J.; Bieri, T.; et al. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature 2003, 423, 825–837. [Google Scholar] [CrossRef]
- Turner, M.E.; Ely, D.; Prokop, J.; Milsted, A. Sry, more than testis determination? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R561–R571. [Google Scholar] [CrossRef]
- Prokop, J.W.; Watanabe, I.K.; Turner, M.E.; Underwood, A.C.; Martins, A.S.; Milsted, A. From rat to human: Regulation of Renin-Angiotensin system genes by sry. Int. J. Hypertens. 2012, 2012, 724240. [Google Scholar] [CrossRef]
- Arain, F.A.; Kuniyoshi, F.H.; Abdalrhim, A.D.; Miller, V.M. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ. J. 2009, 73, 1774–1782. [Google Scholar] [CrossRef]
- Becker, R.C.; Terrin, M.; Ross, R.; Knatterud, G.L.; Desvigne-Nickens, P.; Gore, J.M.; Braunwald, E. Comparison of clinical outcomes for women and men after acute myocardial infarction. The Thrombolysis in Myocardial Infarction Investigators. Ann. Intern. Med. 1994, 120, 638–645. [Google Scholar] [CrossRef]
- Blauwet, L.A.; Redberg, R.F. The role of sex-specific results reporting in cardiovascular disease. Cardiol. Rev. 2007, 15, 275–278. [Google Scholar] [CrossRef]
- Kannel, W.B.; Sorlie, P.; McNamara, P.M. Prognosis after initial myocardial infarction: The Framingham study. Am. J. Cardiol. 1979, 44, 53–59. [Google Scholar] [CrossRef]
- King, K.M.; Ghali, W.A.; Faris, P.D.; Curtis, M.J.; Galbraith, P.D.; Graham, M.M.; Knudtson, M.L. Sex differences in outcomes after cardiac catheterization: Effect modification by treatment strategy and time. JAMA 2004, 291, 1220–1225. [Google Scholar] [CrossRef]
- Mallik, S.; Vaccarino, V. Outcomes of thrombolytic therapy for acute myocardial infarction in women. Prog. Cardiovasc. Dis. 2004, 47, 58–71. [Google Scholar] [CrossRef]
- Shaw, L.J.; Bairey Merz, C.N.; Pepine, C.J.; Reis, S.E.; Bittner, V.; Kelsey, S.F.; Olson, M.; Johnson, B.D.; Mankad, S.; Sharaf, B.L.; et al. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J. Am. Coll. Cardiol. 2006, 47, S4–S20. [Google Scholar] [CrossRef]
- D Bloomer, L.D.; Nelson, C.P.; Eales, J.; Denniff, M.; Christofidou, P.; Debiec, R.; Moore, J.; Consortium, C.; Zukowska-Szczechowska, E.; Goodall, A.H.; et al. Male-specific region of the Y chromosome and cardiovascular risk: Phylogenetic analysis and gene expression studies. Arter. Thromb. Vasc. Biol. 2013, 33, 1722–1727. [Google Scholar] [CrossRef]
- Case, L.K.; Wall, E.H.; Dragon, J.A.; Saligrama, N.; Krementsov, D.N.; Moussawi, M.; Zachary, J.F.; Huber, S.A.; Blankenhorn, E.P.; Teuscher, C. The Y chromosome as a regulatory element shaping immune cell transcriptomes and susceptibility to autoimmune disease. Genome Res. 2013, 23, 1474–1485. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.K.; Oparil, S.; Imthurn, B.; Jackson, E.K. Sex hormones and hypertension. Cardiovasc. Res. 2002, 53, 688–708. [Google Scholar] [CrossRef] [Green Version]
- De Vries, G.J.; Rissman, E.F.; Simerly, R.B.; Yang, L.Y.; Scordalakes, E.M.; Auger, C.J.; Swain, A.; Lovell-Badge, R.; Burgoyne, P.S.; Arnold, A.P. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. The Journal of neuroscience: J. Neurosci. 2002, 22, 9005–9014. [Google Scholar] [CrossRef]
- Ji, H.; Zheng, W.; Wu, X.; Liu, J.; Ecelbarger, C.M.; Watkins, R.; Arnold, A.P.; Sandberg, K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension 2010, 55, 1275–1282. [Google Scholar] [CrossRef]
- Miller, J.A.; Anacta, L.A.; Cattran, D.C. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999, 55, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Sampson, A.K.; Moritz, K.M.; Jones, E.S.; Flower, R.L.; Widdop, R.E.; Denton, K.M. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008, 52, 666–761. [Google Scholar] [CrossRef]
- Tatchum-Talom, R.; Eyster, K.M.; Martin, D.S. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can. J. Physiol. Pharmacol. 2005, 83, 413–422. [Google Scholar] [CrossRef]
- Sampson, A.K.; Jennings, G.L.; Chin-Dusting, J.P. Y are males so difficult to understand? A case where “X” does not mark the spot. Hypertension 2012, 59, 525–531. [Google Scholar] [CrossRef]
- Ely, D.L.; Turner, M.E. Hypertension in the spontaneously hypertensive rat is linked to the Y chromosome. Hypertension 1990, 16, 277–281. [Google Scholar] [CrossRef]
- Davidson, A.O.; Schork, N.; Jaques, B.C.; Kelman, A.W.; Sutcliffe, R.G.; Reid, J.L.; Dominiczak, A.F. Blood pressure in genetically hypertensive rats. Influence of the Y chromosome. Hypertension 1995, 26, 452–459. [Google Scholar] [CrossRef]
- Negrín, C.D.; McBride, M.W.; Carswell, H.V.; Graham, D.; Carr, F.J.; Clark, J.S.; Jeffs, B.; Anderson, N.H.; Macrae, I.M.; Dominiczak, A.F. Reciprocal consomic strains to evaluate y chromosome effects. Hypertension 2001, 37, 391–397. [Google Scholar] [CrossRef]
- Ely, D.; Turner, M.; Milsted, A. Review of the Y chromosome and hypertension. Braz J. Med. Biol. Res. 2000, 33, 679–691. [Google Scholar] [CrossRef]
- Ely, D.L.; Falvo, J.; Dunphy, G.; Caplea, A.; Salisbury, R.; Turner, M. The spontaneously hypertensive rat Y chromosome produces an early testosterone rise in normotensive rats. J. Hypertens. 1994, 12, 769–774. [Google Scholar] [CrossRef]
- Milsted, A.; Underwood, A.C.; Dunmire, J.; DelPuerto, H.L.; Martins, A.S.; Ely, D.L.; Turner, M.E. Regulation of multiple renin-angiotensin system genes by Sry. J. Hypertens. 2010, 28, 59–64. [Google Scholar] [CrossRef]
- Sampson, A.K.; Andrews, K.L.; Graham, D.; McBride, M.W.; Head, G.A.; Thomas, M.C.; Chin-Dusting, J.P.; Dominiczak, A.F.; Jennings, G.L. Origin of the Y chromosome influences intrarenal vascular responsiveness to angiotensin I and angiotensin (1–7) in stroke-prone spontaneously hypertensive rats. Hypertension 2014, 64, 1376–1383. [Google Scholar] [CrossRef]
- Ely, D.; Caplea, A.; Dunphy, G.; Daneshvar, H.; Turner, M.; Milsted, A.; Takiyyudin, M. Spontaneously hypertensive rat Y chromosome increases indexes of sympathetic nervous system activity. Hypertension 1997, 29, 613–618. [Google Scholar] [CrossRef]
- Wiley, D.H.; Dunphy, G.; Daneshvar, H.; Salisbury, R.; Neeki, M.; Ely, D.L. Neonatal sympathectomy reduces adult blood pressure and cardiovascular pathology in Y chromosome consomic rats. Blood Press. 1999, 8, 300–307. [Google Scholar]
- Chen, X.; McClusky, R.; Chen, J.; Beaven, S.W.; Tontonoz, P.; Arnold, A.P.; Reue, K. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet 2012, 8, e1002709. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Loh, D.H.; Colwell, C.S.; Taché, Y.; Reue, K.; Arnold, A.P. Sex differences in diurnal rhythms of food intake in mice caused by gonadal hormones and complement of sex chromosomes. Horm. Behav. 2015, 75, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Bonthuis, P.J.; Rissman, E.F. Neural growth hormone implicated in body weight sex differences. Endocrinology 2013, 154, 3826–3835. [Google Scholar] [CrossRef]
- Link, J.C.; Chen, X.; Prien, C.; Borja, M.S.; Hammerson, B.; Oda, M.N.; Arnold, A.P.; Reue, K. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arter. Thromb. Vasc. Biol. 2015, 35, 1778–1786. [Google Scholar] [CrossRef]
- Suto, J.-I.; Satou, K. Effect of the Y chromosome on plasma high-density lipoprotein-cholesterol levels in Y-chromosome-consomic mouse strains. BMC Res. Notes 2014, 7, 393. [Google Scholar] [CrossRef]
- Strahorn, P.; Graham, D.; Charchar, F.J.; Sattar, N.; McBride, M.W.; Dominiczak, A.F. Genetic determinants of metabolic syndrome components in the stroke-prone spontaneously hypertensive rat. J. Hypertens. 2005, 23, 2179–2186. [Google Scholar] [CrossRef]
- Khan, S.I.; Andrews, K.L.; Jefferis, A.M.; Jennings, G.L.; Sampson, A.K.; Chin-Dusting, J.P.F. Vascular dysfunction in the stroke-prone spontaneously hypertensive rat is dependent on constrictor prostanoid activity and Y chromosome lineage. Clin. Sci. (Lond) 2018, 132, 131–143. [Google Scholar] [CrossRef]
- Khan, S.I.; Andrews, K.L.; Jackson, K.L.; Memon, B.; Jefferis, A.M.; Lee, M.K.S.; Diep, H.; Wei, Z.; Drummond, G.R.; Head, G.A.; et al. Y-chromosome lineage determines cardiovascular organ T-cell infiltration in the stroke-prone spontaneously hypertensive rat. FASEB J. 2018, 32, 2747–2756. [Google Scholar] [CrossRef]
- Ely, D.; Boehme, S.; Dunphy, G.; Hart, M.; Chiarappa, F.; Miller, B.; Martins, A.S.; Turner, M.; Milsted, A. The Sry3 Y chromosome locus elevates blood pressure and renin-angiotensin system indexes. Gend. Med. 2011, 8, 126–138. [Google Scholar] [CrossRef]
- Milsted, A.; Serova, L.; Sabban, E.L.; Dunphy, G.; Turner, M.E.; Ely, D.L. Regulation of tyrosine hydroxylase gene transcription by Sry. Neurosci. Lett. 2004, 369, 203–207. [Google Scholar] [CrossRef]
- Ely, D.; Milsted, A.; Dunphy, G.; Boehme, S.; Dunmire, J.; Hart, M.; Toot, J.; Turner, M. Delivery of sry1, but not sry2, to the kidney increases blood pressure and sns indices in normotensive wky rats. BMC Physiol. 2009, 9, 10. [Google Scholar] [CrossRef]
- Sirianni, R.; Nogueira, E.; Bassett, M.H.; Carr, B.R.; Suzuki, T.; Pezzi, V.; Andò, S.; Rainey, W.E. The AP-1 family member FOS blocks transcriptional activity of the nuclear receptor steroidogenic factor 1. J. Cell Sci. 2010, 123, 3956–3965. [Google Scholar] [CrossRef] [Green Version]
- Stubbins, R.E.; Najjar, K.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab. 2012, 14, 58–66. [Google Scholar] [CrossRef]
- Charchar, F.J.; Tomaszewski, M.; Lacka, B.; Zakrzewski, J.; Zukowska-Szczechowska, E.; Grzeszczak, W.; Dominiczak, A.F. Association of the human Y chromosome with cholesterol levels in the general population. Arter. Thromb. Vasc. Biol. 2004, 24, 308–312. [Google Scholar] [CrossRef]
- Uehara, Y.; Shin, W.S.; Watanabe, T.; Osanai, T.; Miyazaki, M.; Kanase, H.; Taguchi, R.; Sugano, K.; Toyo-Oka, T. A hypertensive father, but not hypertensive mother, determines blood pressure in normotensive male offspring through body mass index. J. Hum. Hypertens. 1998, 12, 441–445. [Google Scholar] [CrossRef]
- Ellis, J.A.; Stebbing, M.; Harrap, S.B. Association of the human Y chromosome with high blood pressure in the general population. Hypertension 2000, 36, 731–733. [Google Scholar] [CrossRef]
- Charchar, F.J.; Tomaszewski, M.; Padmanabhan, S.; Lacka, B.; Upton, M.N.; Inglis, G.C.; Anderson, N.H.; McConnachie, A.; Zukowska-Szczechowska, E.; Grzeszczak, W.; et al. The Y chromosome effect on blood pressure in two European populations. Hypertension 2002, 39, 353–356. [Google Scholar] [CrossRef]
- Shankar, R.R.; Charchar, F.J.; Eckert, G.J.; Saha, C.; Tu, W.; Dominiczak, A.F.; Pratt, J. Studies of an association in boys of blood pressure and the Y chromosome. Am. J. Hypertens. 2007, 20, 27–31. [Google Scholar] [CrossRef]
- Charchar, F.J.; Bloomer, L.D.; Barnes, T.A.; Cowley, M.J.; Nelson, C.P.; Wang, Y.; Denniff, M.; Debiec, R.; Christofidou, P.; Nankervis, S.; et al. Inheritance of coronary artery disease in men: An analysis of the role of the Y chromosome. Lancet 2012, 379, 915–922. [Google Scholar] [CrossRef]
- Voskarides, K.; Hadjipanagi, D.; Papazachariou, L.; Griffin, M.; Panayiotou, A.G. Evidence for contribution of the y chromosome in atherosclerotic plaque occurrence in men. Genet. Test Mol. Biomark. 2014, 18, 552–556. [Google Scholar] [CrossRef]
- Haitjema, S.; van Setten, J.; Eales, J.; van der Laan, S.W.; Gandin, I.; de Vries, J.P.; de Borst, G.J.; Pasterkamp, G.; Asselbergs, F.W.; Charchar, F.J.; et al. Genetic variation within the Y chromosome is not associated with histological characteristics of the atherosclerotic carotid artery or aneurysmal wall. Atherosclerosis 2017, 259, 114–119. [Google Scholar] [CrossRef]
- Hiura, Y.; Fukushima, Y.; Kokubo, Y.; Okamura, T.; Goto, Y.; Nonogi, H.; Takahashi, R.; Iwai, N. Effects of the Y chromosome on cardiovascular risk factors in Japanese men. Hypertens. Res. 2008, 31, 1687–1694. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R.; Lerman, A., Jr. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef]
- Schachinger, V.; Britten, M.B.; Zeiher, A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation 2000, 101, 1899–1906. [Google Scholar] [CrossRef]
- Halcox, J.P.; Schenke, W.H.; Zalos, G.; Mincemoyer, R.; Prasad, A.; Waclawiw, M.A.; Nour, K.R.; Quyyumi, A.A. Prognostic value of coronary vascular endothelial dysfunction. Circulation 2002, 106, 653–658. [Google Scholar] [CrossRef]
- Yeboah, J.; Crouse, J.R.; Hsu, F.C.; Burke, G.L.; Herrington, D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: The Cardiovascular Health Study. Circulation 2007, 115, 2390–2397. [Google Scholar] [CrossRef]
- Gokce, N.; Keaney, J.F.; Jr Hunter, L.M.; Watkins, M.T.; Menzoian, J.O.; Vita, J.A. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function: A prospective study. Circulation 2002, 105, 1567–1572. [Google Scholar] [CrossRef]
- Miyakawa, A.A.; de Lourdes Junqueira, M.; Krieger, J.E. Identification of two novel shear stress responsive elements in rat angiotensin I converting enzyme promoter. Physiol. Genom. 2004, 17, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Vanhoutte Paul, M. Endothelium-Dependent Contractions in Hypertension. Hypertension 2011, 57, 526–531. [Google Scholar] [CrossRef] [Green Version]
- Aken, B.L.; Ayling, S.; Barrell, D.; Clarke, L.; Curwen, V.; Fairley, S.; Fernandez Banet, J.; Billis, K.; García Girón, C.; Hourlier, T.; et al. The Ensembl gene annotation system. Database (Oxford) 2016, 2016, baw093. [Google Scholar] [CrossRef]
- Kruidenier, L.; Chung, C.W.; Cheng, Z.; Liddle, J.; Che, K.; Joberty, G.; Bantscheff, M.; Bountra, C.; Bridges, A.; Diallo, H.; et al. A selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response. Nature 2012, 488, 404–408. [Google Scholar] [CrossRef] [Green Version]
- Vogt, M.H.; Goulmy, E.; Kloosterboer, F.M.; Blokland, E.; de Paus, R.A.; Willemze, R.; Falkenburg, J.H. UTY gene codes for an HLA-B60-restricted human male-specific minor histocompatibility antigen involved in stem cell graft rejection: Characterization of the critical polymorphic amino acid residues for T-cell recognition. Blood 2000, 96, 3126–3132. [Google Scholar]
- Medbury, H.J.; Williams, H.; Fletcher, J.P. Clinical significance of macrophage phenotypes in cardiovascular disease. Clin. Transl. Med. 2014, 3, 63. [Google Scholar] [CrossRef]
- Sezgin, E.; Lind, J.M.; Shrestha, S.; Hendrickson, S.; Goedert, J.J.; Donfield, S.; Kirk, G.D.; Phair, J.P.; Troyer, J.L.; O’Brien, S.J.; et al. Association of Y chromosome haplogroup I with HIV progression, and HAART outcome. Hum. Genet. 2009, 125, 281–294. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Hoch, N.E.; Brown, K.A.; McCann, L.A.; Rahman, A.; Dikalov, S.; Goronzy, J.; Weyand, C.; Harrison, D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007, 204, 2449–2460. [Google Scholar] [CrossRef]
- Crowley, S.D.; Song, Y.S.; Lin, E.E.; Griffiths, R.; Kim, H.S.; Ruiz, P. Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1089–R1097. [Google Scholar] [CrossRef]
- Wei, Z.; Spizzo, I.; Diep, H.; Drummond, G.R.; Widdop, R.E.; Vinh, A. Differential phenotypes of tissue-infiltrating T cells during angiotensin II-induced hypertension in mice. PLoS ONE 2014, 9, e114895. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Quiroz, Y.; Ferrebuz, A.; Parra, G.; Vaziri, N.D. Evolution of renal interstitial inflammation and NF-kappaB activation in spontaneously hypertensive rats. Am. J. Nephrol. 2004, 24, 587–594. [Google Scholar] [CrossRef]
- Carnevale, D.; Pallante, F.; Fardella, V.; Fardella, S.; Iacobucci, R.; Federici, M.; Cifelli, G.; De Lucia, M.; Lembo, G. The Angiogenic Factor PlGF Mediates a Neuroimmune Interaction in the Spleen to Allow the Onset of Hypertension. Immunity 2014, 41, 737–752. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Iturbe, B. Renal infiltration of immunocompetent cells: Cause and effect of sodium-sensitive hypertension. Clin. Exp. Nephrol. 2010, 14, 105–111. [Google Scholar] [CrossRef]
- Kvakan, H.; Kleinewietfeld, M.; Qadri, F.; Park, J.K.; Fischer, R.; Schwarz, I.; Rahn, H.P.; Plehm, R.; Wellner, M.; Elitok, S.; et al. Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 2009, 119, 2904–2912. [Google Scholar] [CrossRef]
- Viel, E.C.; Lemarie, C.A.; Benkirane, K.; Paradis, P.; Schiffrin, E.L. Immune regulation and vascular inflammation in genetic hypertension. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H938–H944. [Google Scholar] [CrossRef] [Green Version]
- Lob, H.E.; Marvar, P.J.; Guzik, T.J.; Sharma, S.; McCann, L.A.; Weyand, C.; Gordon, F.J.; Harrison, D.G. Induction of hypertension and peripheral inflammation by reduction of extracellular superoxide dismutase in the central nervous system. Hypertension 2010, 55, 277–283, 6p following 83. [Google Scholar] [CrossRef]
- Tipton, A.J.; Baban, B.; Sullivan, J.C. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R359–R367. [Google Scholar] [CrossRef]
- Brinson, K.N.; Elmarakby, A.A.; Tipton, A.J.; Crislip, G.R.; Yamamoto, T.; Baban, B.; Sullivan, J.C. Female SHR have greater blood pressure sensitivity and renal T cell infiltration following chronic NOS inhibition than males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R701–R710. [Google Scholar] [CrossRef]
- Ji, H.; Zheng, W.; Li, X.; Liu, J.; Wu, X.; Zhang, M.A.; Umans, J.G.; Hay, M.; Speth, R.C.; Dunn, S.E.; et al. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension 2014, 64, 573–582. [Google Scholar] [CrossRef]
- Pollow, D.P.; Uhrlaub, J.; Romero-Aleshire, M.; Sandberg, K.; Nikolich-Zugich, J.; Brooks, H.L.; Hay, M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension 2014, 64, 384–390. [Google Scholar] [CrossRef]
- Girón-González, J.A.; Moral, F.J.; Elvira, J.; García-Gil, D.; Guerrero, F.; Gavilán, I.; Escobar, L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 2000, 143, 31–36. [Google Scholar] [CrossRef] [Green Version]
- Tipton, A.J.; Baban, B.; Sullivan, J.C. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension 2014, 64, 557–564. [Google Scholar] [CrossRef]
- Feletou, M.; Verbeuren, T.J.; Vanhoutte, P.M. Endothelium-dependent contractions in SHR: A tale of prostanoid TP and IP receptors. Br. J. Pharmacol. 2009, 156, 563–574. [Google Scholar] [CrossRef]
| Reference | Study Conducted In | Major Results |
|---|---|---|
| Ji et al., 2010 [15] | FCG mouse model | Presence of Y chromosome is associated with blunted pressor response to angiotensin II |
| Ely and Turner, 1990 [20] | SHR consomic strains | First study to show that there is a significant BP locus on the SHR Y chromosome. |
| Davidson et al., 1995 [21] | SHRSP consomic strains | First study to show significant BP locus on the SHRSP Y chromosome |
| Negrin et al., 2001 [22] | SHRSP consomic strains | Y chromosome lineage influences salt sensitivity |
| Ely et al., 2000 [23] | SHR consomic strains | Y chromosome lineage influences coronary collagen deposition |
| Ely et al., 2000 [23] | SHR consomic strains | Y chromosome influences renal norepinephrine turnover |
| Ely et al., 1995 [24] | SHR consomic strains | SHR Y chromosome mediates an early testosterone rise |
| Ely et al., 2011 [25] | SHR consomic strains | Sry3 upregulates angiotensinogen, renin and ACE promoter activity in vitro and mediates an increase in sodium reabsorption |
| Sampson et al., 2014 [26] | SHRSP consomic strains | Origin of Y chromosome influences intrarenal vascular responsiveness to RAS peptides |
| Ely et al., 1997 [27] | SHR consomic strains | Y chromosome lineage influences indexes of SNS activity |
| Wiley et al., 1999 [28] | SHRSP consomic strains | Sympathectomy abolishes BP differences between WKY and WKY.SPGlaY |
| Chen et al., 2012 [29] | FCG mouse model | XX chromosome complement mediates increased adiposity and metabolic disturbances in mice fed HFD |
| Chen et al., 2015 [30] | FCG mouse model | XX chromosome complement accounts for increased food intake in mice on HFD |
| Bonthuis et al., 2013 [31] | FCG mouse model | XX chromosome complement increases growth hormone gene in preoptic area of hypothalamus |
| Link et al., 2015 [32] | FCG mouse model | XX chromosome complement accounts for elevated HDL levels |
| Suto and Satou et al., 2019 [33] | Y consomic mouse strains | Y chromosome lineage accounts for differences in lipoprotein profiles |
| Strahorn et al., 2005 [34] | SHRSP consomic strains | Y chromosome lineage determines metabolic phenotypes, mediated in part via an interaction with chromosome 2 |
| Khan et al., 2018 [35] | SHRSP consomic strains | Y chromosome lineage accounts for vascular dysfunction in the SHRSP through influencing cyclo-oxygenase activity |
| Khan et al., 2019 [36] | SHRSP consomic strains | Y chromosome lineage determines perivascular and renal T cell infiltration to in turn influence vascular function |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.I.; Andrews, K.L.; Jennings, G.L.; Sampson, A.K.; Chin-Dusting, J.P.F. Y Chromosome, Hypertension and Cardiovascular Disease: Is Inflammation the Answer? Int. J. Mol. Sci. 2019, 20, 2892. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122892
Khan SI, Andrews KL, Jennings GL, Sampson AK, Chin-Dusting JPF. Y Chromosome, Hypertension and Cardiovascular Disease: Is Inflammation the Answer? International Journal of Molecular Sciences. 2019; 20(12):2892. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122892
Chicago/Turabian StyleKhan, Shanzana I., Karen L. Andrews, Garry L. Jennings, Amanda K. Sampson, and Jaye P. F. Chin-Dusting. 2019. "Y Chromosome, Hypertension and Cardiovascular Disease: Is Inflammation the Answer?" International Journal of Molecular Sciences 20, no. 12: 2892. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20122892