Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals
Abstract
:1. Carbonic Anhydrase and Its Biological Function
1.1. Carbonic Anhydrase
1.2. Biological Function
2. Untangling Global and Local Impacts
2.1. Global Impacts
2.2. Local Impacts
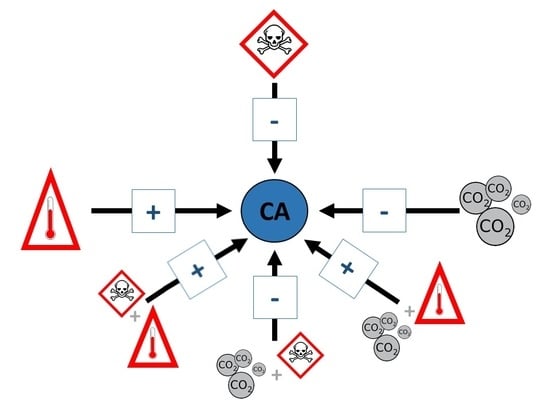
2.3. Tangled Effects: The Role of Interactions
3. Bioindicators and Biomarkers
3.1. Bioindicators
3.2. Biomarkers
4. Carbonic Anhydrase as a Biomarker in Calcifying Organisms
4.1. Global Impacts: Effects of Water Acidification
4.2. Global Impacts: Effects of Warming
4.3. Local Impacts: Effects of Environmental Pollution
4.4. Combined Impacts: The Role of Interactions
5. Corals in the Spotlight of Biomonitoring Programs: Combining Carbonic Anhydrase Assessment with Specific Organismal Analyses
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tripp, B.C.; Smith, K.S.; Ferry, J.G. Carbonic Anhydrase: New Insights for an Ancient Enzyme. J. Biol. Chem. 2001, 276, 48615–48618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Roy, N.; Jackson, D.J.; Marie, B.; Ramos-Silva, P.; Marin, F. Carbonic anhydrase and metazoan biocalcification: A focus on molluscs. Key Eng. Mater. 2016, 672, 151–157. [Google Scholar] [CrossRef]
- Christianson, D.W.; Fierke, C.A. Carbonic Anhydrase: Evolution of the zinc binding site by nature and by design. Acc. Chem. Res. 1996, 29, 331–339. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Gilmour, K.M.; Perry, S.F. Carbonic anhydrase and acid-base regulation in fish. J. Exp. Biol. 2009, 212, 1647–1661. [Google Scholar] [CrossRef] [PubMed]
- Monserrat, J.M.; Martínez, P.E.; Geracitano, L.A.; Amado, L.L.; Martins, C.M.G.; Pinho, G.L.L.; Chaves, I.S.; Ferreira-Cravo, M.; Ventura-Lima, J.; Bianchini, A. Pollution biomarkers in estuarine animals: Critical review and new perspectives. Comp. Biochem. Physiol. C 2007, 146, 221–234. [Google Scholar] [CrossRef]
- Henry, R.P.; Cameron, J.N. The distribution and partial characterization of carbonic anhydrase in selected aquatic and terrestrial decapod crustaceans. J. Exp. 1982, 221, 309–321. [Google Scholar] [CrossRef]
- Henry, R.P. The role of carbonic anhydrase in blood ion and acid-base regulation. Am. Zool. 1984, 24, 241–251. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Grosell, M. Intestinal anion exchange in marine fish osmoregulation. J. Exp. Biol. 2006, 209, 2813–2827. [Google Scholar] [CrossRef] [Green Version]
- Grosell, M.; Genz, J.; Taylor, J.R.; Perry, S.F.; Gilmour, K.M. The involvement of H+-ATPase and carbonic anhydrase in intestinal HCO3− secretion in seawater acclimated rainbow trout. J. Exp. Biol. 2009, 212, 1940–1948. [Google Scholar] [CrossRef] [PubMed]
- Grosell, M.; Laliberte, C.N.; Wood, S.; Jensen, F.B.; Wood, C.M. Intestinal HCO3− secretion in marine teleost fish: Evidence for an apical rather than a basolateral Cl−/HCO3− exchanger. Fish. Physiol. Biochem. 2001, 24, 81–95. [Google Scholar] [CrossRef]
- Grosell, M.; Wood, C.M.; Wilson, R.W.; Bury, N.R.; Hogstrand, C.; Rankin, C.; Jensen, F.B. Bicarbonate secretion plays a role in chloride and water absorption of the European flounder intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R936–R946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genz, J.; Taylor, J.R.; Grosell, M. Effects of salinity on intestinal bicarbonate secretion and compensatory regulation of acid–base balance in Opsanus Beta. J. Exp. Biol. 2008, 211, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Burnett, L.E. CO2 excretion across isolated perfused crab gills: Facilitation by carbonic anhydrase. Am. Zool. 1984, 24, 253–264. [Google Scholar] [CrossRef]
- McMahon, B.R.; Burnett, L.E.; Defur, P.L. Carbon dioxide excretion and carbonic anhydrase function in the red rock crab, Cancer productus. J. Comp. Physiol. 1984, 154, 371–383. [Google Scholar] [CrossRef]
- Weihrauch, D.; Wilkie, M.P.; Walsh, P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2009, 212, 1716–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonnerholm, C. Pulmonary carbonic anhydrase in the human, monkey and rat. J. Appl. Physiol. 1982, 52, 352–356. [Google Scholar] [CrossRef]
- Karlmark, B.B.; Agerup, B.; Wistrand, P.J. Renal proximal tubular acidification. Role of brush-border and cytoplasmic carbonic anhydrase. Acta Physiol. Scud 1979, 106, 145–150. [Google Scholar] [CrossRef]
- Elder, J.A.; Lehninger, A.L. Respiration-dependent transport of carbon dioxide into rat liver mitochondria. Biochemistry 1973, 12, 976–982. [Google Scholar] [CrossRef]
- Simone, G.; Supuran, C.T. Antiobesity carbonic anhydrase inhibitors. Curr. Top. Med. Chem. 2007, 7, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Colombo-Pallotta, M.F.; Rodríguez-Román, A.; Iglesias-Prieto, R. Calcification in bleached and unbleached Montastraea faveolata: Evaluating the role of oxygen and glycerol. Coral Reefs 2010, 29, 899–907. [Google Scholar] [CrossRef]
- Wild, C.; Hoegh-Guldberg, O.; Naumann, M.S.; Colombo-Pallotta, M.F.; Ateweberhan, M.; Fitt, W.K.; Iglesiasprieto, R.; Palmer, C.; Bythell, J.C.; Ortiz, J.C.; et al. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 2011, 62, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Allemand, D.; Ferrier-Pagès, C.; Furla, P.; Houlbrèque, F.; Puverel, S.; Reynaud, S.; Tambutté, É.; Tambutté, S.; Zoccola, D. Biomineralisation in reef-building corals: From molecular mechanisms to environmental control. Gen. Palaeont. 2004, 3, 453–467. [Google Scholar] [CrossRef]
- Al-Horani, F.A.; Al-Moghrabi, S.M.; De Beer, D. The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 2003, 142, 419–426. [Google Scholar] [CrossRef]
- Zoccola, D.; Tambutte, E.; Kulhanek, E.; Puverel, S.; Scimeca, J.C.; Allemand, D.; Tambutte, S. Molecular cloning and localization of a PMCA P-type calcium ATPase from the coral Stylophora Pist. Biochim. Biophys. Acta-Biomembr. 2004, 1663, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Bertucci, A.; Moya, A.; Tambutté, S.; Allemand, D.; Supuran, C.T.; Zoccola, D. Carbonic anhydrases in anthozoan corals e a review. Bioorg. Med. Chem. 2013, 21, 1437–1450. [Google Scholar] [CrossRef] [PubMed]
- Sandeman, I.M. Light driven lipid peroxidation of coral membranes and a suggested role in calcification. Rev. Biol. Trop. 2008, 56, 1–9. [Google Scholar] [CrossRef]
- Pedrozo, H.A.; Schwartz, Z.; Dean, D.D.; Wiederhold, M.L.; Boyan, B.D. Regulation of statoconia mineralization in Aplysia californica in vitro. Connect. Tissue Res. 1996, 35, 317–323. [Google Scholar] [CrossRef]
- Ebanks, S.C.; O’Donnell, M.J.; Grosell, M. Acquisition of Ca2+ and HCO3−/CO3− for shell formation in embryos of the common pond snail Lymnaea stagnalis. J. Comp. Physiol. B 2010, 180, 953–965. [Google Scholar] [CrossRef]
- Gaume, B.M.; Fouchereau-Peron, A.; Badou, M.N.; Helléouet, S.; Auzoux-Bordenave, S. Biomineralization markers during early shell formation in the European abalone Haliotis tuberculata, Linnaeus. Mar. Biol. 2011, 158, 341–353. [Google Scholar] [CrossRef]
- Jones, W.C.; Ledger, P.W. The effect of diamox and various concentrations of calcium on spicule secretion in the calcareous sponge Sycon Cibiatum. Comp. Biochem. Physiol. 1986, 84, 149–158. [Google Scholar] [CrossRef]
- Lucas, J.M.; Knapp, L.W. A physiological evalution of carbon sources for calcification in the octocoral Leptogorgia virgulata (Lamarck). J. Exp. Biol. 1997, 200, 2653–2662. [Google Scholar] [PubMed]
- Marangoni, L.F.B.; Calderon, E.N.; Marques, J.A.; Pereira, C.M.; Duarte, G.A.S.; Castro, C.B.; Bianchini, A. Effects of CO2-driven acidification of seawater on the calcification process in the calcareous hydrozoan Millepora alcicornis (Linnaeus, 1758). Coral Reefs 2017, 36, 1133–1141. [Google Scholar] [CrossRef]
- Kingsley, R.; Watabe, N. Role of carbonic anhydrase in calcification in the gorgonian Leptogorgia Virgulata. J. Exp. Zool. 1987, 241, 171–180. [Google Scholar] [CrossRef]
- Giraud, M.M. Carbonic anhydrase activity in the integument of the crab Carcinus maenas during the intermolt cycle. Comp. Biochem. Physiol. 1981, 69, 381–387. [Google Scholar] [CrossRef]
- Giraud-Guille, M.M. Calcification initiation sites in the crab cuticle: The interprismatic septa. An ultrastructural cytochemical study. Cell Tissue Res. 1984, 236, 413–420. [Google Scholar] [CrossRef]
- Okazaki, M. Carbonic anhydrase in the calcareous red alga, Serraticardia Maxima. Bot. Mar. 1972, 15, 133–138. [Google Scholar] [CrossRef]
- Zilberberg, C.; Abrantes, D.P.; Marques, J.A.; Machado, L.F.; Marangoni, L.F.B. Conhecendo os Recifes Brasileiros: Rede de Pesquisas Coral Vivo; Museu Nacional: Rio de Janeiro, UFRJ, Brasil, 2016; 360p. [Google Scholar]
- IPCC. The Fifth Assessment Report of the Intergovernmental Panel on Climate. 2014. Available online: https://www.ipcc.ch/assessment-report/ar5/ (accessed on 20 February 2019).
- Uprety, D.C.; Reddy, V.R.; Mura, J.D. Greenhouse Gases: A Historical Perspective. In Climate Change and Agriculture; Springer: Singapore, 2019; pp. 31–41. [Google Scholar]
- Rodhe, H. A comparison of the contribution of various gases to the greenhouse effect. Science 1990, 248, 1217–1219. [Google Scholar] [CrossRef]
- Kroeker, K.J.; Kordas, R.L.; Crim, R.; Hendriks, I.E.; Ramajo, L.; Singh, G.S.; Gattuso, J.P. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Chang. Biol. 2013, 19, 1884–1896. [Google Scholar] [CrossRef]
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.; Frieler, K.; Knutti, R.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 C. Nature 2009, 458, 1158. [Google Scholar] [CrossRef]
- Cook, J.; Oreskes, N.; Doran, P.T.; Anderegg, W.R.; Verheggen, B.; Maibach, E.W.; Nuccitelli, D. Consensus on consensus: A synthesis of consensus estimates on human-caused global warming. Environ. Res. Lett. 2016, 11, 048002. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57. [Google Scholar] [CrossRef]
- Baroiller, J.F.; D’Cotta, H.; Saillant, E. Environmental effects on fish sex determination and differentiation. Sex. Dev. 2009, 3, 118–135. [Google Scholar] [CrossRef]
- Inazawa, J.; Hattori, R.S.; Oura, M.; Yokota, M.; Strüssmann, C.A. Temperature effects on sex differentiation of the reciprocal hybrids of Odontesthes bonariensis and Odontesthes hatcheri (Atherinopsidae). Aquac. Res. 2011, 42, 746–753. [Google Scholar] [CrossRef]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Cherkasov, A.S.; Biswas, P.K.; Ridings, D.M.; Ringwood, A.H.; Sokolova, I.M. Effects of acclimation temperature and cadmium exposure on cellular energy budgets in a marine mollusk Crassostrea virginica: Linking cellular and mitochondrial responses. J. Exp. Biol. 2006, 209, 1274–1284. [Google Scholar] [CrossRef]
- Madeira, D.; Narciso, L.; Cabral, H.N.; Vinagre, C.; Diniz, M.S. Influence of temperature in thermal and oxidative stress responses in estuarine fish. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2013, 166, 237–243. [Google Scholar] [CrossRef]
- Fonseca, J.S.; Marangoni, L.F.; Marques, J.A.; Bianchini, A. Effects of increasing temperature alone and combined with copper exposure on biochemical and physiological parameters in the zooxanthellate scleractinian coral Mussismilia Harttii. Aquat. Toxicol. 2017, 190, 121–132. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Yu, Z.X.; Yao, C.L. The impact of acute thermal stress on green mussel Perna viridis: Oxidative damage and responses. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2018, 222, 7–15. [Google Scholar] [CrossRef]
- Zafalon-Silva, B.; Zebral, Y.D.; Bianchini, A.; Da Rosa, C.E.; Marins, L.F.; Colares, E.P.; Robaldo, R.B. Erythrocyte nuclear abnormalities and leukocyte profile in the Antarctic fish Notothenia coriiceps after exposure to short-and long-term heat stress. Pol. Biol. 2017, 40, 1755–1760. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Zebral, Y.D.; Roza, M.; da Silva Fonseca, J.; Costa, P.G.; Oliveira, C.S.; Zocke, T.G.; Bianchini, A. Waterborne copper is more toxic to the killifish Poecilia vivipara in elevated temperatures: Linking oxidative stress in the liver with reduced organismal thermal performance. Aquat. Toxicol. 2019, 209, 142–149. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; Warner, R.R. Global trajectories of the long-term decline of coral reef ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef]
- Magrin, G.O.; Marengo, J.A.; Boulanger, J.-P.; Buckeridge, M.S.; Castellanos, E.; Poveda, G.; Scarano, F.R.; Vicuña, S. 2014: Central and South America. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part. B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 1499–1566. [Google Scholar]
- Wilkinson, C.R. Global and local threats to coral reef functioning and existence: Review and predictions. Mar. Freshw. Res. 1999, 50, 867–878. [Google Scholar] [CrossRef]
- Borgå, K. Ecotoxicology: Bioaccumulation. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 346–348. [Google Scholar]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Lewitus, A. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Goldscheider, N. Delineation of spring protection zones. In Groundwater Hydrology of Springs; Elsevier Butterworth-Heinemann: Oxford, UK, 2010; pp. 305–338. [Google Scholar]
- Carpenter, K.E.; Abrar, M.; Aeby, G.; Aronson, R.B.; Banks, S.; Bruckner, A.; Edgar, G.J. One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 2008, 321, 560–563. [Google Scholar] [CrossRef]
- Ban, S.S.; Graham, N.A.; Connolly, S.R. Evidence for multiple stressor interactions and effects on coral reefs. Glob. Chang. Biol. 2014, 20, 681–697. [Google Scholar] [CrossRef]
- Knowlton, N.; Jackson, J.B.C. Shifting Baselines, Local Impacts, and Global Change on Coral Reefs. PLoS Biol. 2008, 6, e54. [Google Scholar] [CrossRef]
- Colwell, R.K.; Coddington, J.A. Estimating terrestrial biodiversity through extrapolation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1994, 345, 101–118. [Google Scholar] [Green Version]
- Rushton, S.P.; Luff, M.L.; Eyre, M.D. Effect of pasture improvement and management on the ground beetle and spider communities of upland grasslands. J. Appl. Ecol. 1989, 26, 489–503. [Google Scholar] [CrossRef]
- Rushton, S.P.; Eyre, M.D.; Luff, M.L. The effects of management on the occurrence of some ground beetle species in grassland. In The Role of Ground Beetles in Ecological and Environmental Studies; Stork, N.E., Ed.; Intercept: Andover, UK, 1990; pp. 209–216. [Google Scholar]
- Abensperg, T.M.; Smith, G.T.; Arnold, G.W.; Steven, D.E. The effects of habitat fragmentation and livestock-grazing on animal communities in remnants of gimlet Eucalyptus salubris woodland in the Western Australian wheatbelt. I. Arthropods. J. Appl. Ecol. 1996, 33, 1281–1303. [Google Scholar] [CrossRef]
- Vieira, C.E.D.; Costa, P.G.; Caldas, S.S.; Tesser, M.E.; Risso, W.E.; Escarrone, A.L.V.; Reis Martinez, C.B. An integrated approach in subtropical agro-ecosystems: Active biomonitoring, environmental contaminants, bioaccumulation, and multiple biomarkers in fish. Sci. Total Environ. 2019, 666, 508–524. [Google Scholar] [CrossRef]
- Marques, D.S.; Costa, P.G.; Souza, G.M.; Cardozo, J.G.; Barcarolli, I.F.; Bianchini, A. Selection of biochemical and physiological parameters in the croaker Micropogonias furnieri as biomarkers of chemical contamination in estuaries using a generalized additive model (GAM). Sci. Total Environ. 2019, 647, 1456–1467. [Google Scholar] [CrossRef]
- Acott, C. JS Haldane, JBS Haldane, L Hill and A Siebe: A brief resume of their lives. South Pac. Underw. Med. Soc. J. 1999, 29, 161–165. [Google Scholar]
- Withrow, S.J.; Vail, D.M. Withrow and MacEwen’s Small Animal Clinical Oncology, 4th ed.; Elsevier: Philadelphia, PA, USA, 2007; pp. 73–74. [Google Scholar]
- Rainio, J.; Niemelä, J. Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodivers. Conserv. 2003, 12, 487–506. [Google Scholar] [CrossRef]
- Cooper, T.F.; Gilmour, J.P.; Fabricius, K.E. Bioindicators of changes in water quality on coral reefs: Review and recommendations for monitoring programmes. Coral Reefs 2009, 28, 589–606. [Google Scholar] [CrossRef]
- Lambeck, R.J. Focal species: A multi-species umbrella for nature conservation. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Hook, S.E.; Gallagher, E.P.; Batley, G.E. The role of biomarkers in the assessment of aquatic ecosystem health. Integr. Environ. Assess. Manag. 2014, 10, 327–341. [Google Scholar] [CrossRef]
- Lionetto, M.G.; Caricato, R.; Erroi, E.; Giordano, M.E.; Schettino, T. Potential application of carbonic anhydrase activity in bioassay and biomarker studies. Chem. Ecol. 2006, 22, S119–S125. [Google Scholar] [CrossRef]
- Kawahata, H.; Fujita, K.; Iguchi, A.; Inoue, M.; Iwasaki, S.; Kuroyanagi, A.; Toyofuku, T. Perspective on the response of marine calcifiers to global warming and ocean acidification—Behavior of corals and foraminifera in a high CO2 world “hot house”. Prog. Earth Planet. Sci. 2019, 6, 5. [Google Scholar] [CrossRef]
- Vogel, N.; Meyer, F.W.; Wild, C.; Uthicke, S. Decreased light availability can amplify negative impacts of ocean acidification on calcifying coral reef organisms. Mar. Ecol. Prog. Ser. 2015, 521, 49–61. [Google Scholar] [CrossRef]
- Spalding, C.; Finnegan, S.; Fischer, W.W. Energetic costs of calcification under ocean acidification. Glob. Biogeochem. Cycles 2017, 31, 866–877. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, L.C.; Straub, S.; Bischof, K. Elevated CO2 levels affect the activity of nitrate reductase and carbonic anhydrase in the calcifying rhodophyte Corallina officinalis. J. Exp. Bot. 2013, 64, 899–908. [Google Scholar] [CrossRef]
- Marangoni, L.F.B.; Pinto, M.M.D.A.N.; Marques, J.A.; Bianchini, A. Copper exposure and seawater acidification interaction: Antagonistic effects on biomarkers in the zooxanthellate scleractinian coral Mussismilia harttii. Aquat. Toxicol. 2019, 206, 123–133. [Google Scholar] [CrossRef]
- Prazeres, M.; Uthicke, S.; Pandolfi, J.M. Ocean acidification induces biochemical and morphological changes in the calcification process of large benthic foraminifera. Proc. R. Soc. B 2015, 282. [Google Scholar] [CrossRef]
- Moya, A.; Huisman, L.; Ball, E.E.; Hayward, D.C.; Grasso, L.C.; Chua, C.M.; Woo, H.N.; Gattuso, J.P.; ForêT, S.; Miller, D.J. Whole transcriptome analysis of the coral Acropora millepora reveals complex responses to CO2-driven acidification during the initiation of calcification. Mol. Ecol. 2012, 21, 2440–2454. [Google Scholar] [CrossRef]
- Zoccola, D.; Innocenti, A.; Bertucci, A.; Tambutté, E.; Supuran, C.; Tambutté, S. Coral carbonic anhydrases: Regulation by ocean acidification. Mar. Drugs 2016, 14, 109. [Google Scholar] [CrossRef]
- Richier, S.; Fiorini, S.; Kerros, M.E.; Von Dassow, P.; Gattuso, J.P. Response of the calcifying coccolithophore Emiliania huxleyi to low pH/high pCO2: From physiology to molecular level. Mar. Biol. 2011, 158, 551–560. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M.; Jia, Z.; Qiu, L.; Wang, L.; Zhang, A.; Song, L. A carbonic anhydrase serves as an important acid-base regulator in pacific oyster Crassostrea gigas exposed to elevated CO2: Implication for physiological responses of mollusk to ocean acidification. Mar. Biotechnol. 2017, 19, 22–35. [Google Scholar] [CrossRef]
- Carreiro-Silva, M.; Cerqueira, T.; Godinho, A.; Caetano, M.; Santos, R.S.; Bettencourt, R. Molecular mechanisms underlying the physiological responses of the cold-water coral Desmophyllum dianthus to ocean acidification. Coral Reefs 2014, 33, 465–476. [Google Scholar] [CrossRef]
- Vidal-Dupiol, J.; Zoccola, D.; Tambutté, E.; Grunau, C.; Cosseau, C.; Smith, K.M.; Freitag, M.; Dheilly, N.M.; Allemand, D.; Tambutté, S. Genes related to ion-transport and energy production are upregulated in response to CO2-driven pH decrease in corals: New insights from transcriptome analysis. PLoS ONE 2013, 8, e58652. [Google Scholar] [CrossRef]
- Sun, T.; Tang, X.; Zhou, B.; Wang, Y. Comparative studies on the effects of seawater acidification caused by CO2 and HCl enrichment on physiological changes in Mytilus edulis. Chemosphere 2016, 144, 2368–2376. [Google Scholar] [CrossRef]
- Fitzer, S.C.; Phoenix, V.R.; Cusack, M.; Kamenos, N.A. Ocean acidification impacts mussel control on biomineralisation. Sci. Rep. 2014, 4, 6218. [Google Scholar] [CrossRef]
- Moreira, A.; Figueira, E.; Soares, A.M.; Freitas, R. The effects of arsenic and seawater acidification on antioxidant and biomineralization responses in two closely related Crassostrea species. Sci. Total Environ. 2016, 545, 569–581. [Google Scholar] [CrossRef]
- Edge, S.E.; Morgan, M.B.; Gleason, D.F.; Snell, T.W. Development of a coral cDNA array to examine gene expression profiles in Montastraea faveolata exposed to environmental stress. Mar. Pollut. Bull. 2005, 51, 507–523. [Google Scholar] [CrossRef]
- Ogawa, D.; Bobeszko, T.; Ainsworth, T.; Leggat, W. The combined effects of temperature and CO 2 lead to altered gene expression in Acropora aspera. Coral Reefs 2013, 32, 895–907. [Google Scholar] [CrossRef]
- Hoadley, K.D.; Pettay, D.T.; Grottoli, A.G.; Cai, W.J.; Melman, T.F.; Schoepf, V.; Matsui, Y. Physiological response to elevated temperature and pCO2 varies across four Pacific coral species: Understanding the unique host+ symbiont response. Sci. Rep. 2015, 5, 18371. [Google Scholar] [CrossRef]
- Ivanina, A.V.; Dickinson, G.H.; Matoo, O.B.; Bagwe, R.; Dickinson, A.; Beniash, E.; Sokolova, I.M. Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comp. Biochem. Physiol. Part. A Mol. Integr. Physiol. 2013, 166, 101–111. [Google Scholar] [CrossRef]
- Pepper, I.L.; Brusseau, M.L.; Gerba, C.P. Environmental and Pollution Science, 3rd ed.; Academic Press/Elsevier: San Diego, CA, USA, 2019. [Google Scholar]
- El-Gendy, K.S.; Radwan, M.A.; Gad, A.F.; Khamis, A.E.; Eshra, E.S.H. Physiological traits of land snails Theba pisanaas simple endpoints to assess the exposure to some pollutants. Environ. Sci. Pollut. Res. 2019, 1–9. [Google Scholar]
- Santini, O.; Chahbane, N.; Vasseur, P.; Frank, H. Effects of low-level copper exposure on Ca2+-ATPase and carbonic anhydrase in the freshwater bivalve Anodonta anatina. Toxicol. Environ. Chem. 2011, 93, 1826–1837. [Google Scholar] [CrossRef]
- Bielmyer, G.K.; Grosell, M.; Bhagooli, R.; Baker, A.C.; Langdon, C.; Gillette, P.; Capo, T.R. Differential effects of copper on three species of scleractinian corals and their algal symbionts (Symbiodinium spp.). Aquat. Toxicol. 2010, 97, 125–133. [Google Scholar] [CrossRef]
- Fonseca, S.J.; de Barros Marangoni, L.F.; Marques, J.A.; Bianchini, A. Carbonic anhydrase activity as a potential biomarker for acute exposure to copper in corals. Chemosphere 2019, 227, 598–605. [Google Scholar] [CrossRef]
- Caricato, R.; Lionetto, M.G.; Dondero, F.; Viarengo, A.; Schettino, T. Carbonic anhydrase activity in Mytilus galloprovincialis digestive gland: Sensitivity to heavy metal exposure. Comp. Biochem. Physiol. C 2010, 152, 241–247. [Google Scholar]
- Balbi, T.; Camisassi, G.; Montagna, M.; Fabbri, R.; Franzellitti, S.; Carbone, C.; Canesi, L. Impact of cationic polystyrene nanoparticles (PS-NH2) on early embryo development of Mytilus galloprovincialis: Effects on shell formation. Chemosphere 2017, 186, 1–9. [Google Scholar] [CrossRef]
- Capolupo, M.; Franzellitti, S.; Valbonesi, P.; Lanzas, C.S.; Fabbri, E. Uptake and transcriptional effects of polystyrene microplastics in larval stages of the Mediterranean mussel Mytilus galloprovincialis. Environ. Pollut. 2018, 241, 1038–1047. [Google Scholar] [CrossRef]
- Santos, M.B.; Neto, I.E.M.; de Souza Melo, S.R.C.; Amado, E.M. Hemolymph and gill carbonic anhydrase are more sensitive to aquatic contamination than mantle carbonic anhydrase in the mangrove oyster Crassostrea rhizophorae. Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2017, 201, 19–25. [Google Scholar] [CrossRef]
- Azevedo-Linhares, M.; Freire, C. Evaluation of impacted Brazilian estuaries using the native oyster Crassostrea rhizophorae: Branchial carbonic anhydrase as a biomarker. Ecotoxicol. Environ. Saf. 2015, 122, 483–489. [Google Scholar] [CrossRef]
- Bielmyer-Fraser, G.K.; Patel, P.; Capo, T.; Grosell, M. Physiological responses of corals to ocean acidification and copper exposure. Mar. Pollut. Bull. 2018, 133, 781–790. [Google Scholar] [CrossRef]
- Kaniewska, P.; Chan, C.K.K.; Kline, D.; Ling, E.Y.S.; Rosic, N.; Edwards, D.; Dove, S. Transcriptomic changes in coral holobionts provide insights into physiological challenges of future climate and ocean change. PLoS ONE 2015, 10, e0139223. [Google Scholar] [CrossRef]
- Obura, D.O. Can differential bleaching and mortality among coral species offer useful indicators for assessment and management of reefs under stress? Bull. Mar. Sci. 2001, 69, 421–442. [Google Scholar]
- Siebeck, U.E.; Marshall, N.J.; Klueter, A.; Hoegh-Guldberg, O. Monitoring coral bleaching using a colour reference card. Coral Reefs 2006, 25, 453–460. [Google Scholar] [CrossRef]
- Bythell, J.C.; Brown, B.E.; Kirkwood, T.B. Do reef corals age? Biol. Rev. 2018, 93, 1192–1202. [Google Scholar] [CrossRef]
- Davies, P. Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 1989, 101, 389–395. [Google Scholar] [CrossRef]
- Chisholm, J.R.M.; Gattuso, J.P. Validation of the alkalinity anomaly technique for investigating calcification and photosynthesis in coral reef communities. Limnol. Oceanogr. 1991, 36, 1232–1239. [Google Scholar] [CrossRef]
- Yao, W.; Byrne, R.H. Simplified seawater alkalinity analysis: Use of linear array spectrometers. Deep. Sea. Res. Part. 1 Oceanogr. Res. Pap. 1998, 45, 1383–1392. [Google Scholar] [CrossRef]
- Lough, J.M. Coral calcification from skeletal records revisited. Mar. Ecol. Prog. Ser. 2008, 373, 257–264. [Google Scholar] [CrossRef]
- De’ath, G.; Lough, J.M.; Fabricius, K.E. Declining coral calcification on the Great Barrier Reef. Science 2009, 323, 116–119. [Google Scholar] [CrossRef]
- Cantin, N.E.; Cohen, A.L.; Karnauskas, K.B.; Tarrant, A.M.; McCorkle, D.C. Ocean warming slows coral growth in the central Red Sea. Science 2010, 329, 322–325. [Google Scholar] [CrossRef]
- Crook, E.D.; Cohen, A.L.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 2013, 110, 11044–11049. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.P.; Anderson, K.D.; Connolly, S.R.; Heron, S.F.; Kerry, J.T.; Lough, J.M.; Claar, D.C. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef] [Green Version]
- Stanley, G.D. Photosymbiosis and the evolution of modern coral reefs. Science 2006, 312, 857–858. [Google Scholar] [CrossRef]
- Yellowlees, D.; Rees, T.A.V.; Leggat, W. Metabolic interactions between algal symbionts and invertebrate hosts. Plant. Cell. Environ. 2008, 31, 679–694. [Google Scholar] [CrossRef]
- Baird, A.H.; Marshall, P.A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 237, 133–141. [Google Scholar] [CrossRef]
- Meehan, W.J.; Ostrander, G.K. Coral bleaching: A potential biomarker of environmental stress. J. Toxicol. Environ. HealthPart. A Curr. Issues 1997, 50, 529–552. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zebral, Y.D.; da Silva Fonseca, J.; Marques, J.A.; Bianchini, A. Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals. Int. J. Mol. Sci. 2019, 20, 3092. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20123092
Zebral YD, da Silva Fonseca J, Marques JA, Bianchini A. Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals. International Journal of Molecular Sciences. 2019; 20(12):3092. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20123092
Chicago/Turabian StyleZebral, Yuri Dornelles, Juliana da Silva Fonseca, Joseane Aparecida Marques, and Adalto Bianchini. 2019. "Carbonic Anhydrase as a Biomarker of Global and Local Impacts: Insights from Calcifying Animals" International Journal of Molecular Sciences 20, no. 12: 3092. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20123092