Blockade of STAT3 Signaling Contributes to Anticancer Effect of 5-Acetyloxy-6,7,8,4′-Tetra-Methoxyflavone, a Tangeretin Derivative, on Human Glioblastoma Multiforme Cells
Abstract
:1. Introduction
2. Results
2.1. 5-AcTMF Evoked in Vitro Cytotoxicity and Apoptosis in a Panel of Human GBM Cell Lines
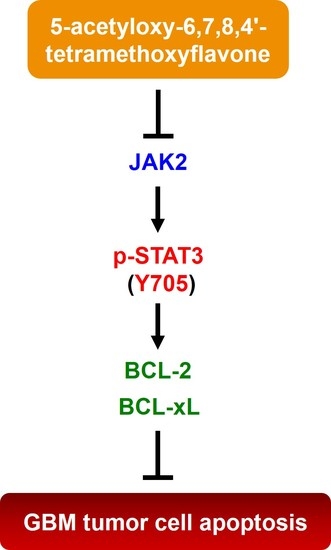
2.2. 5-AcTMF Suppressed both Constitutive and IL-6-Induced STAT3 Activation in Human GBM cells
2.3. Suppression of STAT3 Activation is Essential for 5-AcTMF to Inhibit GBM Cell Survival and Growth
2.4. 5-AcTMF Blocks STAT3 Activation to Downregulate BCL-2 and BCL-xL
2.5. BCL-2 Downregulation is Required for 5-AcTMF to Induce GBM Cell Apoptosis
2.6. BCL-xL Downregulation is Necessary for 5-AcTMF-Induced Apoptosis in Human GBM cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Colony Formation Assay
4.5. Annexin V-FITC Assay
4.6. Construction of pBabe-Based Expressing Plasmids for Generating Stable Clones of HA-STAT3-CA, BCL-2, or HA-BCL-xL
4.7. Retrovirus Production and Infection
4.8. Immunoblotting
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fox, B.M.; Janssen, A.; Estevez-Ordonez, D.; Gessler, F.; Vicario, N.; Chagoya, G.; Elsayed, G.; Sotoudeh, H.; Stetler, W.; Friedman, G.K.; et al. SUMOylation in glioblastoma: A novel therapeutic target. Int. J. Mol. Sci. 2019, 20, 1853. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, G.S.; Dzhenkov, D.; Ghenev, P.; Iliev, B.; Enchev, Y.; Tonchev, A.B. Cell biology of glioblastoma multiforme: From basic science to diagnosis and treatment. Med. Oncol. 2018, 35, 27. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011-2015. Neuro-Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Mitchell, D.A. The development of dendritic cell vaccine-based immunotherapies for glioblastoma. Semin. Immunopathol. 2017, 39, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Ouédraogo, Z.G.; Biau, J.; Kemeny, J.L.; Morel, L.; Verrelle, P.; Chautard, E. Role of STAT3 in genesis and progression of human malignant gliomas. Mol. Neurobiol. 2017, 54, 5780–5797. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Ahn, S.H.; Kong, D.S.; Lee, H.W.; Nam, D.H. The role of STAT3 in glioblastoma progression through dual influences on tumor cells and the immune microenvironment. Mol. Cell. Endocrinol. 2017, 451, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.S.; Yang, L.J.; Wang, X.F.; Chen, Y.P.; Tang, W.L.; Chen, L.; Lin, Z.X. STAT3 Tyr705 phosphorylation affects clinical outcome in patients with newly diagnosed supratentorial glioblastoma. Med. Oncol. 2014, 31, 924. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xue, X.; Zhou, H.; Zhang, G. A molecular view of the radioresistance of gliomas. Oncotarget 2017, 8, 100931–100941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohsaka, S.; Wang, L.; Yachi, K.; Mahabir, R.; Narita, T.; Itoh, T.; Tanino, M.; Kimura, T.; Nishihara, H.; Tanaka, S. STAT3 inhibition overcomes temozolomide resistance in glioblastoma by downregulating MGMT expression. Mol. Cancer Ther. 2012, 11, 1289–1299. [Google Scholar] [CrossRef]
- Masliantsev, K.; Pinel, B.; Balbous, A.; Guichet, P.O.; Tachon, G.; Milin, S.; Godet, J.; Duchesne, M.; Berger, A.; Petropoulos, C.; et al. Impact of STAT3 phosphorylation in glioblastoma stem cells radiosensitization and patient outcome. Oncotarget 2018, 9, 3968–3979. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, D.; Fan, M.; Yang, C.H.; Zbytek, B.; Finkelstein, D.; Roussel, M.F.; Pfeffer, L.M. The critical role that STAT3 plays in glioma-initiating cells: STAT3 addiction in glioma. Oncotarget 2018, 9, 22095–22112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, J.R.D.; Regad, T. Targeting cellular pathways in glioblastoma multiforme. Signal Transduct. Target. Ther. 2017, 2, 17040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brantley, E.C.; Benveniste, E.N. Signal transducer and activator of transcription-3: A molecular hub for signaling pathways in gliomas. Mol. Cancer Res. 2008, 6, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Linder, B.; Weirauch, U.; Ewe, A.; Uhmann, A.; Seifert, V.; Mittelbronn, M.; Harter, P.N.; Aigner, A.; Kögel, D. Therapeutic targeting of Stat3 using lipopolyplex nanoparticle-formulated siRNA in a syngeneic orthotopic mouse glioma model. Cancers 2019, 11, 333. [Google Scholar] [CrossRef]
- Jensen, K.V.; Cseh, O.; Aman, A.; Weiss, S.; Luchman, H.A. The JAK2/STAT3 inhibitor pacritinib effectively inhibits patient-derived GBM brain tumor initiating cells in vitro and when used in combination with temozolomide increases survival in an orthotopic xenograft model. PLoS ONE 2017, 12, e0189670. [Google Scholar] [CrossRef]
- Li, S.; Lo, C.Y.; Dushenkov, S.; Ho, C.T. Polymethoxyflavones: Chemistry, biological activity, and occurrence in orange peel. In Dietary Supplements; Ho, C.T., Simon, J.E., Shahidi, F., Shao, Y., Eds.; ACS Symposium Series; American Chemical Society (ACS): Washington, DC, USA, 2008; Volume 987, Chapter 13; pp. 191–210. [Google Scholar]
- Wang, X.; Li, S.; Wei, C.C.; Huang, J.; Pan, M.H.; Shahidi, F.; Ho, C.-T. Anti-inflammatory effects of polymethoxyflavones from citrus peels: A review. J. Food Bioact. 2018, 3, 76–86. [Google Scholar] [CrossRef]
- Tung, Y.C.; Chou, Y.C.; Hung, W.L.; Cheng, A.C.; Yu, R.C.; Ho, C.T.; Pan, M.H. Polymethoxyflavones: Chemistry and molecular mechanisms for cancer prevention and treatment. Curr. Pharmacol. Rep. 2019. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Tangeretin, a polymethoxylated flavone, modulates lipid homeostasis and decreases oxidative stress by inhibiting NF-κB activation and proinflammatory cytokines in cardiac tissue of streptozotocin-induced diabetic rats. J. Funct. Foods 2015, 16, 315–333. [Google Scholar] [CrossRef]
- Shu, Z.; Yang, B.; Zhao, H.; Xu, B.; Jiao, W.; Wang, Q.; Wang, Z.; Kuang, H. Tangeretin exerts anti-neuroinflammatory effects via NF-κB modulation in lipopolysaccharide-stimulated microglial cells. Int. Immunopharmacol. 2014, 19, 275–282. [Google Scholar] [CrossRef]
- Periyasamy, K.; Baskaran, K.; Ilakkia, A.; Vanitha, K.; Selvaraj, S.; Sakthisekaran, D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7,12-dimethylbenz(a) anthracene-induced proliferative breast cancer in Sprague-Dawley rats. Cancer Chemother. Pharmacol. 2015, 75, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Lai, C.S.; Chung, C.H.; Yang, J.M.; Hsu, K.C.; Chen, C.Y.; Chung, C.S.; Li, S.; Ho, C.T.; Pan, M.H. 5-Demethyltangeretin is more potent than tangeretin in inhibiting dimethylbenz(a)anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin tumorigenesis. J. Funct. Foods 2014, 11, 528–537. [Google Scholar] [CrossRef]
- Wang, J.; Duan, Y.; Zhi, D.; Li, G.; Wang, L.; Zhang, H.; Gu, L.; Ruan, H.; Zhang, K.; Liu, Q.; et al. Pro-apoptotic effects of the novel tangeretin derivate 5-acetyl-6,7,8,4′-tetramethylnortangeretin on MCF-7 breast cancer cells. Cell Biochem. Biophys. 2014, 70, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.S.; Zeng, J.Y.; Li, S.; Huang, Q.; Ho, C.T.; Pan, M.-H. Inhibitory effects of 5-demethyltangeretin and 5-acetyloxy-6,7,8,4′-tetramethoxyflavone on human colon cancer cells. In Nutrition, Functional and Sensory Properties of Foods, 1st ed.; Ho, C.-T., Mussinan, C., Shahidi, F., Contis, E.T., Eds.; The Royal Society of Chemistry: London, UK, 2013; pp. 281–290. [Google Scholar]
- Li, Y.R.; Li, S.; Ho, C.T.; Chang, Y.H.; Tan, K.T.; Chung, T.W.; Wang, B.Y.; Chen, Y.K.; Lin, C.C. Tangeretin derivative, 5-acetyloxy-6,7,8,4′-tetramethoxyflavone induces G2/M arrest, apoptosis and autophagy in human non-small cell lung cancer cells in vitro and in vivo. Cancer Biol. Ther. 2015, 17, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Liu, S.; Lin, L.; Wang, L.; Wang, J.; Ma, J.; Wang, S.; Zhao, H.; Ho, C.-T.; Wang, Y.; et al. 5-Acetyl-6,7,8,4′-tetramethylnortangeretin induces apoptosis in multiple myeloma U266 cells. Food Sci. Hum. Wellness 2014, 3, 197–203. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.H. The polymethoxylated flavone, Tangeretin improves cognitive memory in rats experiencing a single episode of prolonged post-traumatic stress. Anim. Cells Syst. 2018, 22, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Zhao, J.; Chen, Y.; Li, T.; Zhu, R.; Zhu, B.; Zhang, Y. Tangeretin protects human brain microvascular endothelial cells against oxygen-glucose deprivation-induced injury. J. Cell. Biochem. 2019, 120, 4883–4891. [Google Scholar] [CrossRef]
- Braidy, N.; Behzad, S.; Habtemariam, S.; Ahmed, T.; Daglia, M.; Nabavi, S.M.; Sobarzo-Sanchez, E.; Nabavi, S.F. Neuroprotective effects of citrus fruit-derived flavonoids, nobiletin and tangeretin in Alzheimer’s and Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2017, 16, 387–397. [Google Scholar] [CrossRef]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef]
- Okuyama, S.; Miyazaki, K.; Yamada, R.; Amakura, Y.; Yoshimura, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Permeation of polymethoxyflavones into the mouse brain and their effect on MK-801-induced locomotive hyperactivity. Int. J. Mol. Sci. 2017, 18, 489. [Google Scholar] [CrossRef]
- Yuan, G.; Yan, S.F.; Xue, H.; Zhang, P.; Sun, J.T.; Li, G.; Yuan, G.; Yan, S.F.; Xue, H.; Zhang, P.; et al. Cucurbitacin I induces protective autophagy in glioblastoma in vitro and in vivo. J. Biol. Chem. 2014, 289, 10607–10619. [Google Scholar] [CrossRef] [PubMed]
- Luwor, R.B.; Stylli, S.S.; Kaye, A.H. The role of Stat3 in glioblastoma multiforme. J. Clin. Neurosci. 2013, 20, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ichijo, H.; Korsmeyer, S.J. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G2/M. Mol. Cell. Biol. 1999, 19, 8469–8478. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-P.; Li, S.; Chuang, W.-L.; Li, C.-H.; Chen, G.-J.; Chang, C.-C.; Or, C.-H.R.; Lin, P.-Y.; Chang, C.-C. Blockade of STAT3 Signaling Contributes to Anticancer Effect of 5-Acetyloxy-6,7,8,4′-Tetra-Methoxyflavone, a Tangeretin Derivative, on Human Glioblastoma Multiforme Cells. Int. J. Mol. Sci. 2019, 20, 3366. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133366
Cheng Y-P, Li S, Chuang W-L, Li C-H, Chen G-J, Chang C-C, Or C-HR, Lin P-Y, Chang C-C. Blockade of STAT3 Signaling Contributes to Anticancer Effect of 5-Acetyloxy-6,7,8,4′-Tetra-Methoxyflavone, a Tangeretin Derivative, on Human Glioblastoma Multiforme Cells. International Journal of Molecular Sciences. 2019; 20(13):3366. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133366
Chicago/Turabian StyleCheng, Yen-Po, Shiming Li, Wan-Ling Chuang, Chia-Hsuan Li, Guan-Jun Chen, Ching-Chin Chang, Chi-Hung R. Or, Ping-Yi Lin, and Chia-Che Chang. 2019. "Blockade of STAT3 Signaling Contributes to Anticancer Effect of 5-Acetyloxy-6,7,8,4′-Tetra-Methoxyflavone, a Tangeretin Derivative, on Human Glioblastoma Multiforme Cells" International Journal of Molecular Sciences 20, no. 13: 3366. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20133366