Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Nanoparticles
2.2. In Vitro Evaluation of MSN@PDA as a Drug Delivery System
2.2.1. Cellular Uptake of MSN@PDA-COP Particles
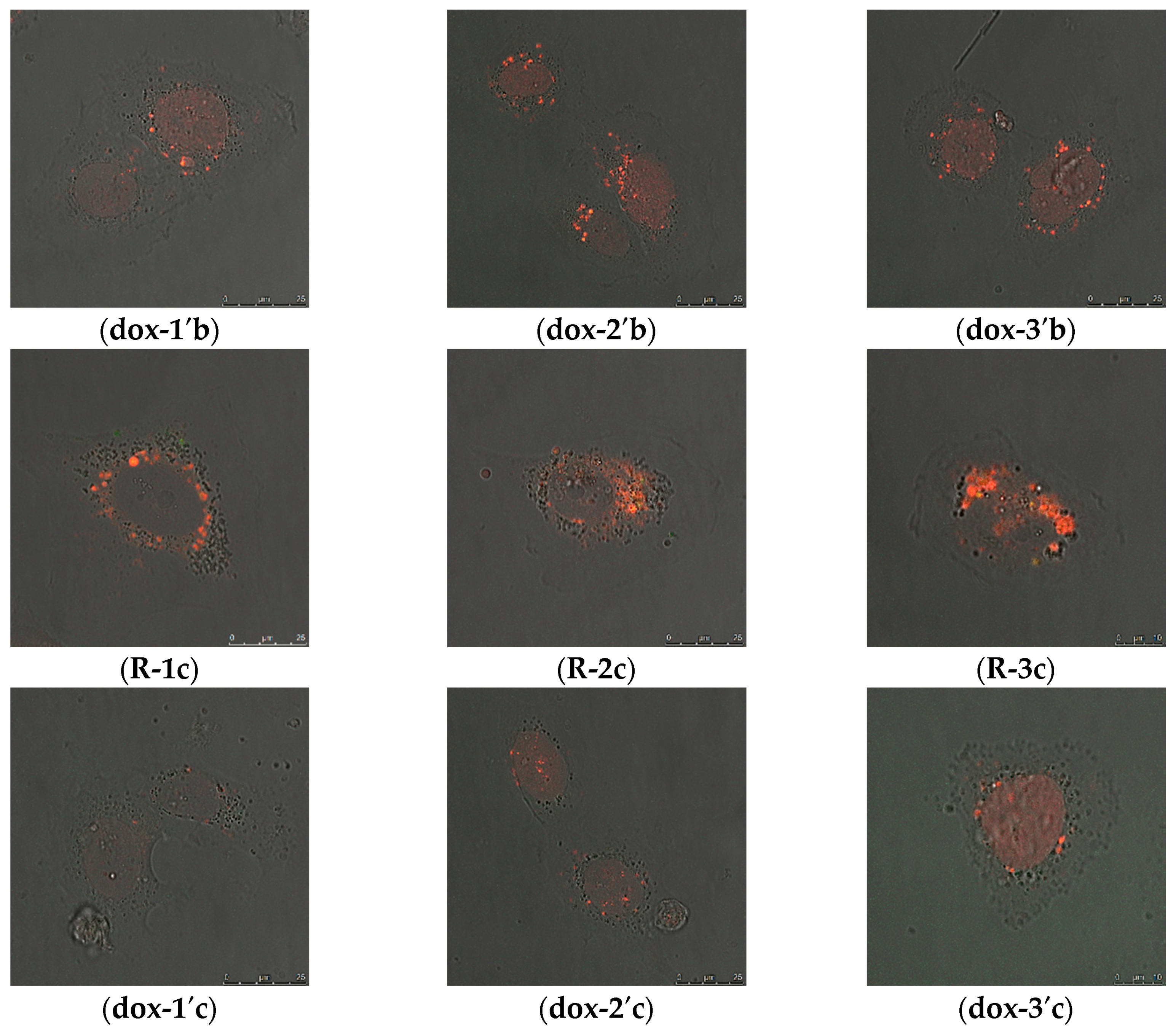
2.2.2. Intracellular Distribution of DOX Delivered by MSN@PDA-COP Particles
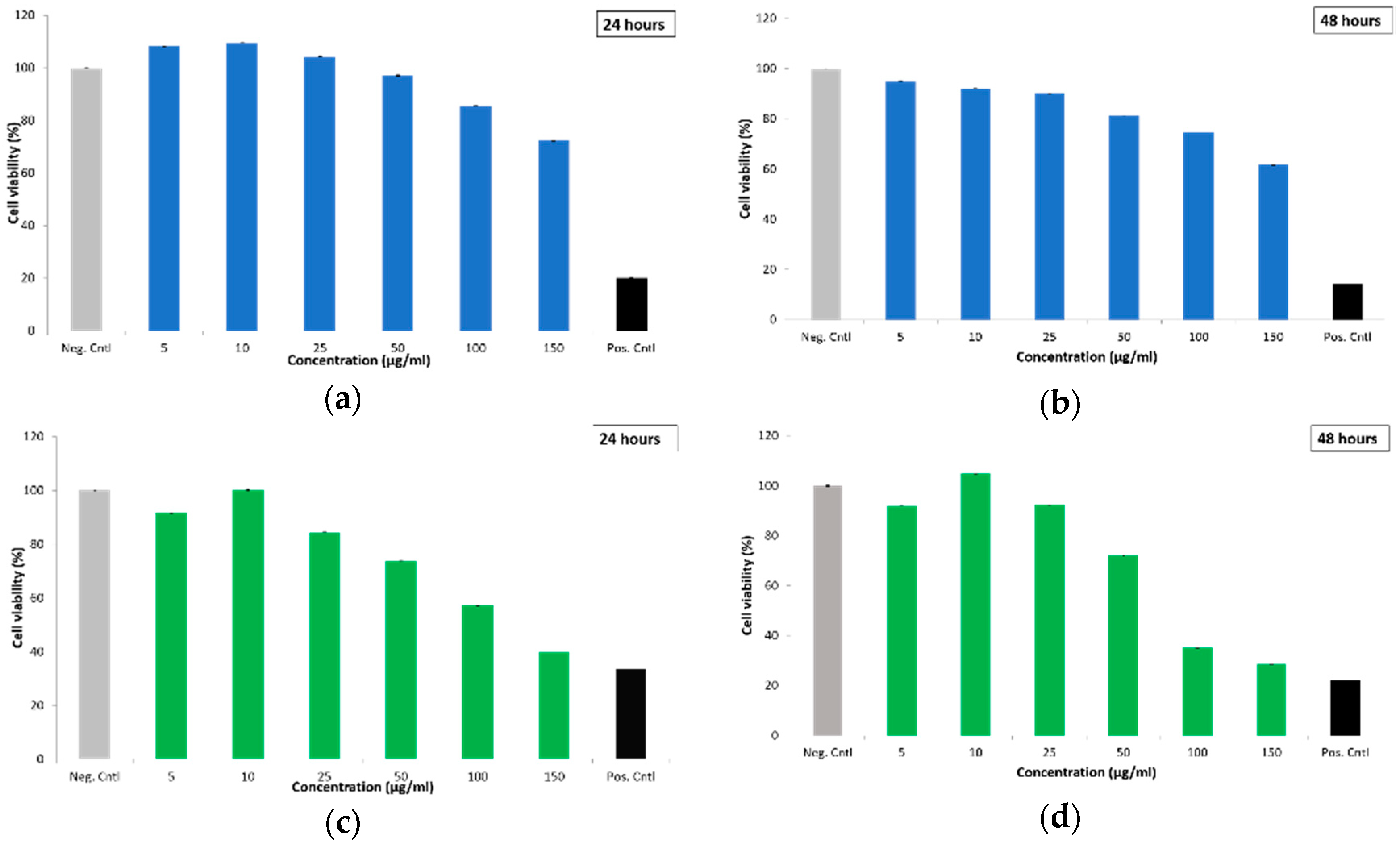
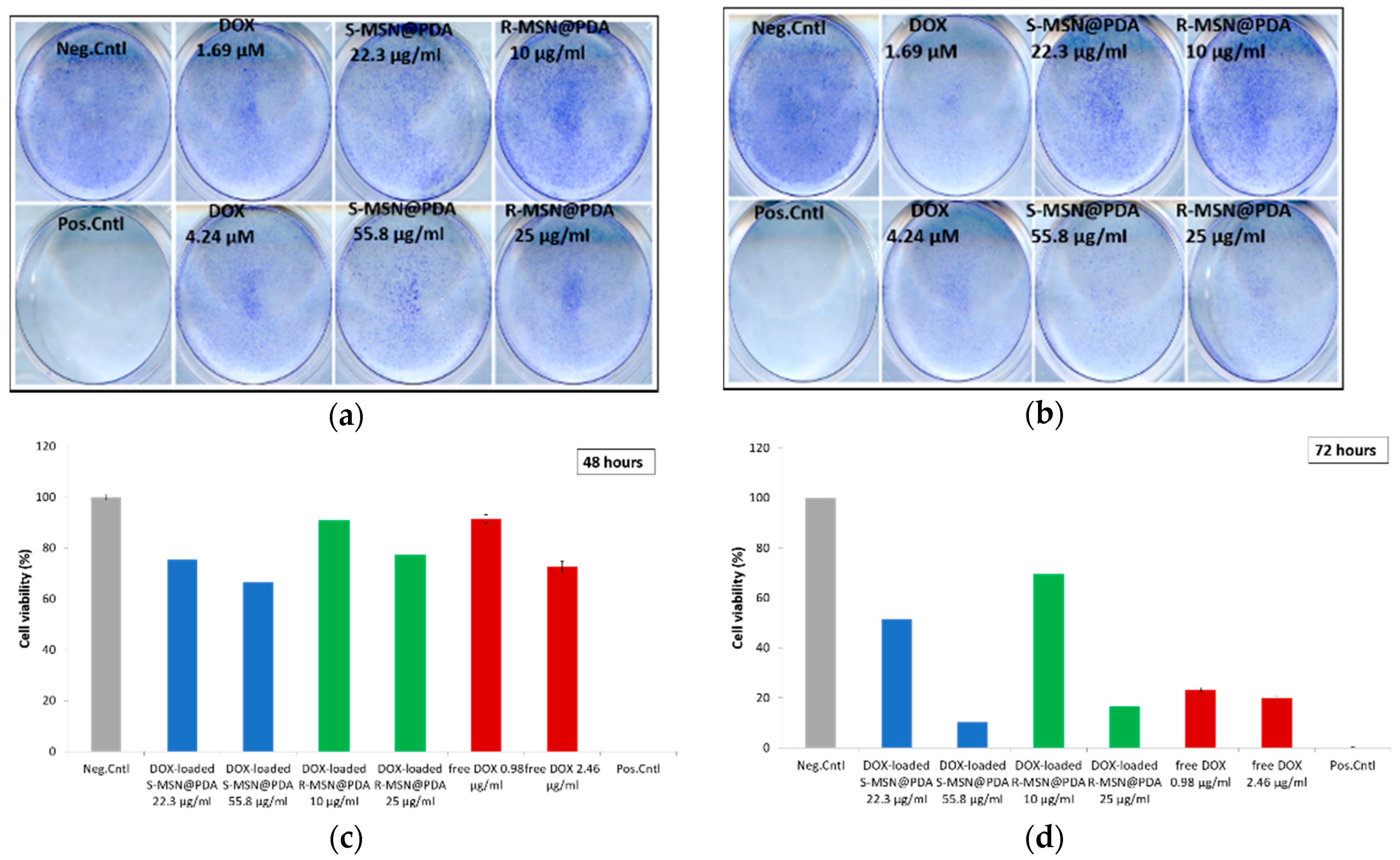
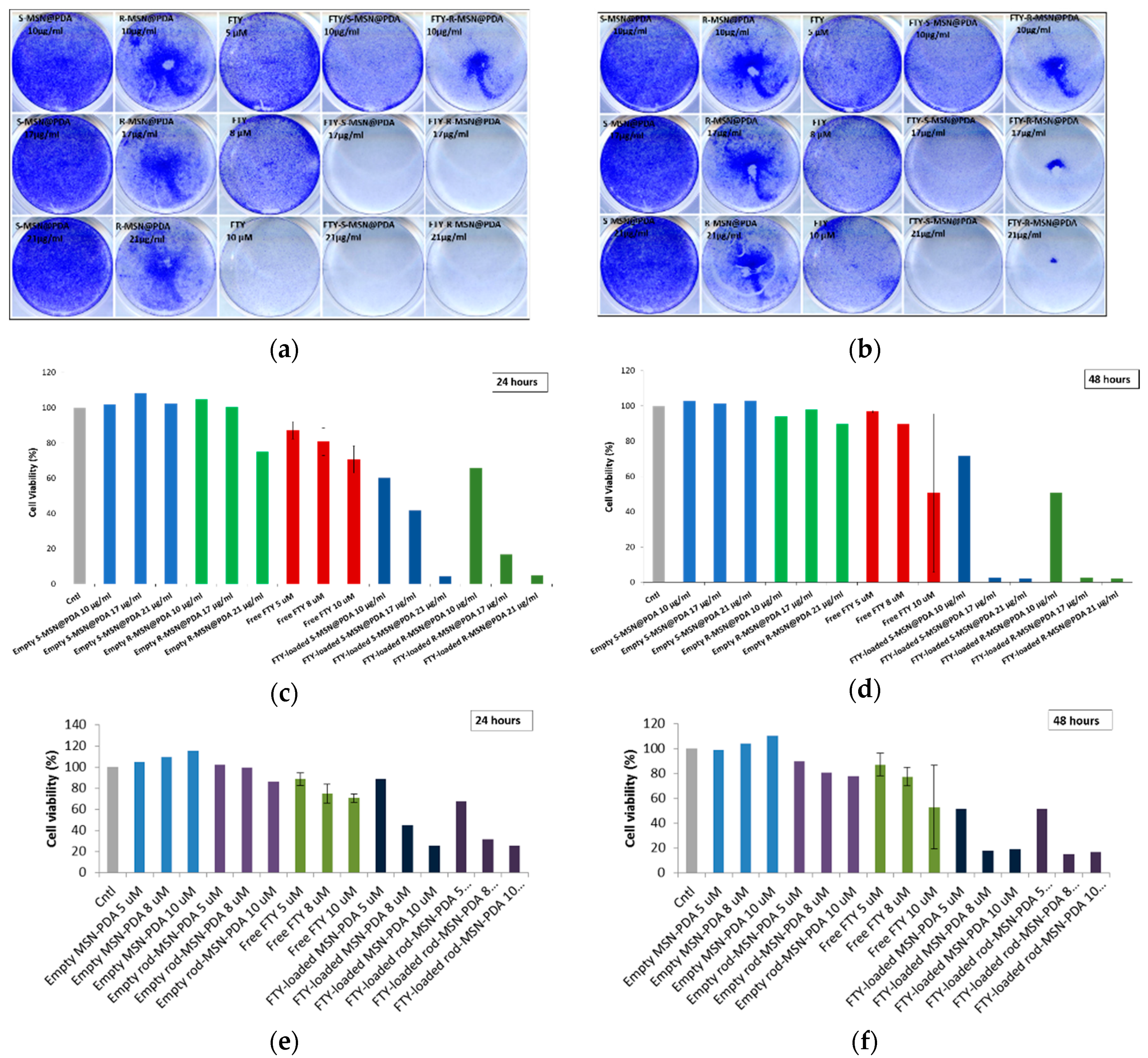
2.2.3. Cytotoxicity of Empty and Drug-Loaded MSN@PDA-COP Particles
3. Materials and Methods
3.1. Polydopamine Coating of MSN
3.2. Characterization of MSN@PDA Particles
3.3. Drug Loading and Loading Degree Determination
3.4. Cell Cultures
3.5. Cellular Uptake of MSN@PDA
3.5.1. Confocal Microscopy
3.5.2. Fluorescent Activated Cell Sorting
3.6. Intracellular Distribution of DOX-Loaded MSN@PDA Particles
3.7. Cytotoxicity
3.7.1. WST-1 for DOX-Loaded Particles
3.7.2. Crystal Violet for DOX-Loaded Particles
3.7.3. Crystal Violet for FTY720-Loaded Particles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MSN | Mesoporous silica nanoparticles |
| PDA | Polydopamine |
| DOX | Doxorubicin |
| FTY720 | Fingolimod |
| MSN-NH2 | Amino-functionalized MSN |
| MDA-MB-231 | Human breast cancer cells |
| ML-1 | Human follicular thyroid cancer cells |
| FACS | Fluorescence Activated Cell Sorting |
| S-MSN | Spherical shaped MSN |
| R-MSN | Rod shaped MSN |
| PEG-PEI | Polyethylene glycol-Polyethylenimine |
| DLS | Dynamic Light Scattering |
| TEM | Transmission Electron Microscopy |
| WST | Water Soluble Tetrazolium salt |
| PI | Propidium Iodide |
| FITC | Fluorescein isothiocyanate |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
References
- Bogunia-Kubik, K.; Sugisaka, M. From Molecular Biology to Nanotechnology and Nanomedicine. Biosystems 2002, 65, 123–138. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Kwak, J.-W.; Park, J.W. Nanotechnology for Early Cancer Detection. Sensors 2010, 10, 428–455. [Google Scholar] [CrossRef] [PubMed]
- Seigneuric, R.; Markey, L.; Nuyten, D.S.A.; Dubernet, C.; Evelo, C.T.A.; Finot, E.; Garrido, C. From Nanotechnology to Nanomedicine: Applications to Cancer Research. Curr. Mol. Med. 2010, 10, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Grobmyer, S.R.; Iwakuma, N.; Sharma, P.; Moudgil, B.M. What Is Cancer Nanotechnology? In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana Press: New York, NY, USA, 2010; Volume 624, pp. 1–9. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Pasqualini, R.; Montalti, M.; Petrizza, L.; Prodi, L.; Rampazzo, E.; Zaccheroni, N.; Marchiò, S. Luminescent Silica Nanoparticles for Cancer Diagnosis. Curr. Med. Chem. 2013, 20, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.-T.; Cheng, S.-H.; Souris, J.S.; Chen, C.-T.; Mou, C.-Y.; Lo, L.-W. Theranostic Applications of Mesoporous Silica Nanoparticles and Their Organic/Inorganic Hybrids. J. Mater. Chem. B 2013, 1, 3128–3135. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, N.; Kim, T.; Kim, J.; Hyeon, T. Multifunctional Mesoporous Silica Nanocomposite Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011, 44, 893–902. [Google Scholar] [CrossRef]
- Ferris, D.P.; Lu, J.; Gothard, C.; Yanes, R.; Thomas, C.R.; Olsen, J.; Stoddart, J.F.; Tamanoi, F.; Zink, J.I. Synthesis of Biomolecule-modified Mesoporous Silica Nanoparticles for Targeted Hydrophobic Drug Delivery to Cancer Cells. Small 2011, 7, 1816–1826. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [Green Version]
- Knežević, N.Ž.; Trewyn, B.G.; Lin, V.S.-Y. Functionalized Mesoporous Silica Nanoparticle-Based Visible Light Responsive Controlled Release Delivery System. Chem. Commun. 2011, 47, 2817–2819. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, X.; Soeriyadi, A.H.; Sagnella, S.M.; Lu, X.; Scott, J.A.; Lowe, S.B.; Kavallaris, M.; Gooding, J.J. Stimuli-Responsive Functionalized Mesoporous Silica Nanoparticles for Drug Release in Response to Various Biological Stimuli. Biomater. Sci. 2014, 2, 121–130. [Google Scholar] [CrossRef]
- Tukappa, A.; Ultimo, A.; de la Torre, C.; Pardo, T.; Sancenón, F.; Martínez-Máñez, R. Polyglutamic Acid-Gated Mesoporous Silica Nanoparticles for Enzyme-Controlled Drug Delivery. Langmuir 2016, 32, 8507–8515. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous Silica Nanoparticles for Stimuli-Responsive Controlled Drug Delivery: Advances, Challenges, and Outlook. Int. J. Nanomed. 2017, 12, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, J.; Wang, J.; Qi, X.; Rosenholm, J.M.; Cai, K. Polydopamine Coatings in Confined Nanopore Space: Toward Improved Retention and Release of Hydrophilic Cargo. J. Phys. Chem. C 2015, 119, 24512–24521. [Google Scholar] [CrossRef]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; De Lange Davies, C.; Sahlgren, C.; et al. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef]
- Huwiler, A.; Zangemeister-Wittke, U. The Sphingosine 1-Phosphate Receptor Modulator Fingolimod as a Therapeutic Agent: Recent Findings and New Perspectives. Pharmacol. Ther. 2018, 185, 34–49. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Gulin-Sarfraz, T.; Hedström, G.; Duchanoy, A.; Eklund, P.; Rosenholm, J.M. Rational Evaluation of the Utilization of PEG-PEI Copolymers for the Facilitation of Silica Nanoparticulate Systems in Biomedical Applications. J. Colloid Interface Sci. 2014, 418, 300–310. [Google Scholar] [CrossRef]
- Kaasalainen, M.; Aseyev, V.; von Haartman, E.; Karaman, D.Ş.; Mäkilä, E.; Tenhu, H.; Rosenholm, J.; Salonen, J. Size, Stability, and Porosity of Mesoporous Nanoparticles Characterized with Light Scattering. Nanoscale Res. Lett. 2017, 12, 74. [Google Scholar] [CrossRef]
- Ferreira, T.; Rasband, W. ImageJ User Guide. In IJ 1.43r; Centre for Research in Neuroscience, McGill University: Montreal, QC, Canada, 2010; pp. 1–187. [Google Scholar]
- Karukstis, K.K.; Thompson, E.H.Z.; Whiles, J.A.; Rosenfeld, R.J. Deciphering the Fluorescence Signature of Daunomycin and Doxorubicin. Biophys. Chem. 1998, 73, 249–263. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin Pathways. Pharm. Genom. 2010, 21, 440–446. [Google Scholar] [CrossRef]
- Jeffery, D.R.; Markowitz, C.E.; Reder, A.T.; Weinstock-Guttman, B.; Tobias, K. Fingolimod for the Treatment of Relapsing Multiple Sclerosis. Expert Rev. Neurother. 2011, 11, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Ingwersen, J.; Aktas, O.; Kuery, P.; Kieseier, B.; Boyko, A.; Hartung, H.P. Fingolimod in Multiple Sclerosis: Mechanisms of Action and Clinical Efficacy. Clin. Immunol. 2012, 142, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, M.A.; Raheja, R.; Murugaiyan, G.; Rajabi, H.; Kumar, D.; Pertel, T.; Regev, K.; Griffin, R.; Aly, L.; Kivisakk, P.; et al. Identification of a Novel Mechanism of Action of Fingolimod (FTY720) on Human Effector T Cell Function through TCF-1 Upregulation. J. Neuroinflammation 2015, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Kalhori, V.; Magnusson, M.; Asghar, M.Y.; Pulli, I.; Törnquist, K. FTY720 (Fingolimod) Attenuates Basal and Sphingosine-1-Phosphate-Evoked Thyroid Cancer Cell Invasion. Endocr. Relat. Cancer 2016, 23, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Li, L.; Tang, F. Shape Matters When Engineering Mesoporous Silica-Based Nanomedicines. Biomater. Sci. 2016, 4, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Karaman, D.S.; Desai, D.; Senthilkumar, R.; Johansson, E.M.; Råtts, N.; Odén, M.; Eriksson, J.E.; Sahlgren, C.; Toivola, D.M.; Rosenholm, J.M. Shape Engineering vs. Organic Modification of Inorganic Nanoparticles as a Tool for Enhancing Cellular Internalization. Nanoscale Res. Lett. 2012, 7, 2–32. [Google Scholar] [CrossRef]
- Shilova, O.N.; Shilov, E.S.; Deyev, S.M. The Effect of Trypan Blue Treatment on Autofluorescence of Fixed Cells. Cytom. Part. A 2017, 91, 917–925. [Google Scholar] [CrossRef]
- Desai, D.; Karaman, D.S.E.N.; Prabhakar, N.; Tadayon, S.; Duchanoy, A.; Toivola, D.M.; Rajput, S.; Näreoja, T.; Rosenholm, J.M. Design Considerations for Mesoporous Silica Nanoparticulate Systems in Facilitating Biomedical Applications. Open Mater. Sci. 2014, 1. [Google Scholar] [CrossRef]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The Effect of the Shape of Mesoporous Silica Nanoparticles on Cellular Uptake and Cell Function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef]
- Zhang, L.; Qiao, S.; Jin, Y.; Cheng, L.; Yan, Z.; Lu, G.Q. Hydrophobic Functional Group Initiated Helical Mesostructured Silica for Controlled Drug Release. Adv. Funct. Mater. 2008, 18, 3834–3842. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Martín Del Valle, E.M. Polydopamine Nanoparticles Kill Cancer Cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef]
- Tacar, O.; Sriamornsak, P.; Dass, C.R. Doxorubicin: An Update on Anticancer Molecular Action, Toxicity and Novel Drug Delivery Systems. J. Pharm. Pharm. 2013, 65, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin Cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.; Russell, R. Anthracycline Cardiotoxicity: Prevalence, Pathogenesis and Treatment. Curr. Cardiol. Rev. 2012, 7, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-Induced Cardiomyopathy: From Molecular Mechanisms to Therapeutic Strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.; Speirs, V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011, 13, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Schönberger, J.; Bauer, J.; Spruss, T.; Weber, G.; Chahoud, I.; Eilles, C.; Grimm, D. Establishment and characterization of the follicular thyroid carcinoma cell line ML-1. J. Mol. Med. (Heidelb. Ger.) 2000, 78, 102–110. [Google Scholar] [CrossRef]





| Particle Type | Z-Average Size (nm) (Measured in DI Water) | Polydispersity Index (PdI) | Zeta Potential (mV) (Measured in HEPES Buffer, 25mM, pH 7.4) |
|---|---|---|---|
| S-MSN | 181.7 ± 1.45 | 0.191 | 15.3 |
| Rod-MSN | 248.73 ± 3.90 | 0.087 | −20.83 ± 1.05 |
| S-MSN@PDA | 191.77 ± 5.15 | 0.269 | −13.43 ± 0.31 |
| Rod-MSN@PDA | 1678.00 ± 159.45 | 0.313 | −22.93 ± 0.96 |
| S-MSN@PDA-COP | 158.80 ± 1.32 | 0.198 | 3.91 ± 0.49 |
| Rod-MSN@PDA-COP | 219.60 ± 4.92 | 0.097 | 5.74 ± 0.39 |
| Particle Type | Concentration (µg/mL) | Uptake by Cells (MFI) |
|---|---|---|
| S-MSN@PDA | 5 | 30 |
| 10 | 40 | |
| 25 | 70 | |
| 50 | 150 | |
| R-MSN@PDA | 5 | 40 |
| 10 | 60 | |
| 25 | 165 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pada, A.-K.; Desai, D.; Sun, K.; Prakirth Govardhanam, N.; Törnquist, K.; Zhang, J.; Rosenholm, J.M. Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs. Int. J. Mol. Sci. 2019, 20, 3408. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20143408
Pada A-K, Desai D, Sun K, Prakirth Govardhanam N, Törnquist K, Zhang J, Rosenholm JM. Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs. International Journal of Molecular Sciences. 2019; 20(14):3408. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20143408
Chicago/Turabian StylePada, Anna-Karin, Diti Desai, Kaiyao Sun, Narayana Prakirth Govardhanam, Kid Törnquist, Jixi Zhang, and Jessica M. Rosenholm. 2019. "Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs" International Journal of Molecular Sciences 20, no. 14: 3408. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20143408






