1. Introduction
Peptide-based synthetic vaccines have attracted a significant amount of attention as a new generation of vaccines, because of their safety benefits and ease of production when compared with that of conventional whole pathogen-based vaccines [
1,
2]. However, poor immune responses are induced when only minimal antigenic epitopes are used without combining suitable adjuvants (immune stimulants), which are sometimes toxic. Nanocarrier-based delivery systems are a promising approach to overcome those drawbacks of peptide vaccines. In designing the nanocarrier, it is important to consider the interaction between the nanocarrier surface and cells, such as antigen presenting cells (APCs).
Over the past few decades, various nanocarriers have been developed, including liposomes [
3,
4,
5], polymeric nanoparticles [
6,
7,
8], and polymeric micelles [
9]. In many of these systems, building block molecules for the construction of nanocarriers are first synthesized and then combined with antigenic peptides via several procedures, including nanomaterial formation and loading of antigenic peptides (encapsulation, chemical immobilization or physical adsorption), to give a nano-formulation. Recently, the use of antigenic peptides that are pre-conjugated to self-assembly motifs has attracted attention as an easier and simpler procedure to produce nano-formulations [
10,
11]. This self-assembly approach ensures highly efficient drug loading without laborious procedures or the use of synthetic components, which sometimes exhibit toxicity. In addition, because the resulting nanostructures consist of a single component, the physicochemical and structural features of these nanostructures can be simply tuned by the design of the building block peptide, and variation in drug loading efficiency among different nanostructures can be eliminated.
Among the various molecular blocks (e.g., lipids [
12,
13,
14] and hydrophobic polymers [
15,
16]) used to assemble antigenic peptides into nanostructures, β-sheet-forming-peptides are extremely attractive because: (i) They can assemble in aqueous solution to give nanofibers (NFs) with highly regulated structures, even when functional molecules with a relatively large molecular weight are conjugated; (ii) the resulting well-ordered β-sheet structures allow the integration of antigens at high density; and (iii) they are relatively easy to synthesize and have high biocompatibility. These advantages make NF-vaccines a good alternative to traditional vaccines. Immune induction by NFs formed from antigenic peptides conjugated to β-sheet-forming-peptides have been reported [
17,
18,
19,
20,
21]. For example, Rudra et al. reported that NFs composed of an antigenic epitope peptide conjugated to self-assembling peptide Q11 were subcutaneously administered, and elicited a strong antibody response [
17]. They have also demonstrated that the β-sheet peptide NF system can be applied to various types of antigens, including a malaria epitope [
18], a
Staphylococcus aureus epitope [
19] and a tumor-associated antigen MUC1 glycopeptide [
20]. However, fundamental studies on how the hydrophilic-hydrophobic balance of NF components affects their cellular interaction—including cellular uptake, cytotoxicity, and immune stimulation response—has not been reported. Recently, studies on other particulate systems reported that surface hydrophobicity is an important factor for determining cellular response [
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32]. In addition to cellular internalization and nontoxicity (i.e., safety), the ability of nanocarriers to stimulate an immune response is an essential property in nanocarrier-based vaccine applications, because uptake of nanocarriers containing an antigen by APCs that do not induce an immune response may lead to unwanted tolerance toward the antigen. Thus, to design nanomaterial-based vaccines that elicit strong immunity without toxicity using a β-sheet assembly system requires a clear understanding of how the hydrophilic-hydrophobic balance of NF components affects their cellular interactions and response.
In previous work, we reported the preparation of antigen-loaded NFs by exploiting the self-assembly of β-sheet peptides [
33,
34] conjugated to antigenic peptides and hydrophilic chains, such as oligo-ethylene glycol (EG) [
35,
36,
37]. MHC class I restricted epitope (SIINFEKL) from ovalbumin was selected as a model antigenic peptide. In addition, the structure of the NFs was analyzed in detail by various techniques, including wide-angle X-ray diffraction (WAXD), small-angle X-ray scattering (SAXS), Fourier transform infrared spectroscopy (FT-IR), circular dichroism (CD), transmission electron microscopy (TEM), and atomic force microscopy (AFM). Interestingly, structural analysis revealed that the shape of the NFs is rectangular, rather than a cylinder-like structure observed for filament micelles, possibly because of the lamination structures of β-sheets. Based on this finding, the structural model was proposed as shown in
Figure 1b, which shows that the surface of the NFs is not covered with EG chains homogeneously [
35,
36]. Thus, we hypothesized that the EG chain length is an important parameter for tuning the cellular interactions of NFs, including cellular uptake, cytotoxicity, and immune stimulation response.
In this study, the effect of the EG chain length in building block molecules, which form peptide NFs, on their cellular interaction was investigated. The self-assembling behavior of three kinds of building block peptides with different EG lengths was evaluated by determining their critical aggregation concentration (CAC) and the critical concentration for nanofiber formation (CFC). The structures of the resulting NFs were analyzed by TEM and CD, and their surface hydrophobicity was evaluated using a hydrophobic fluorescence probe. Cellular uptake, cytotoxicity, and immune stimulation ability of the three kinds of NFs were examined in vitro using DCs. In addition, interaction of cells with micelle-like aggregates that were composed of the same building blocks as the NFs were also investigated. Cellular interaction of the NFs was found to be significantly dependent on EG length, whereas that of micelles was independent of EG length. Notably, uptake by DC of NFs composed of EG with a moderate length was effective, and the NFs activated DC without exhibiting significant cytotoxicity. The findings provide useful design guidelines for the development of effective nanofiber-based vaccines.
3. Discussion
In this study, we have investigated cellular uptake, cytotoxicity, and DC stimulatory activity of antigen-loaded peptide NFs with different EG lengths and their component peptides. Three building block peptide amphiphiles with different EG lengths (6-mer, 12-mer and 24-mer) were prepared. ThT assay, TEM observation, and CD measurement revealed that all type of peptide amphiphiles are successfully formed β-sheet rich nanofibers with distinct widths (
Figure 2,
Figure 3 and
Figure 4). The association state of EG
n peptides was dependent on sample concentration. EG
n peptides self-assembled into NFs above the CFC, formed spherical micelles at concentrations between the CFC and CAC, and existed as monomers in solution below the CAC. Based on these findings, we discuss separately the effect of EG length on cellular uptake, cytotoxicity, and immune stimulation for three peptide states: NFs, micelles, and monomers.
3.1. Effect of EG Length of Nanofibers on Their Cellular Uptake, Cytotoxicity, and Immune Stimulation Ability
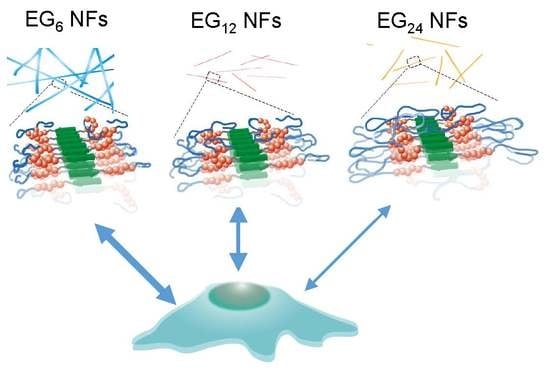
The EG length of NFs significantly affected their cellular uptake, cytotoxicity, and DC stimulatory activity. Here, we discuss the effect of EG length on these properties of NFs using structural models derived from SAXS, WAXD, CD, and FT-IR data of a previous study [
36]. FT-IR, CD, and WAXD results indicate that EG
12 NFs contain β-sheet structures. In addition, synchrotron X-ray scattering profiles of EG
12 NFs revealed that the morphology of the NFs is rectangular, and they do not form cylinder structures like filament micelles, presumably because of the laminated structure of β-sheets. In general, amyloid-like nanofibers have a common characteristic cross-β-sheet structure, where tightly packed β-sheets orientate themselves perpendicularly to the fiber elongation axis [
47]. By combining these findings, we propose a model of EG
n NFs (
Figure S7). β-sheet structures consisting mainly of hydrophobic amino acids form the framework of NFs with EG chains facing outwards to provide water-dispersibility. The surfaces of NFs possess hydrophobic and hydrophilic domains that consist of EG chains based on this model. The ANS assay results support the notion that there are hydrophobic domains on the surface of NFs.
3.1.1. Cellular Association and Internalization of NFs
The amount of NFs associated with cells increased in the order of EG
24 NFs, EG
12 NFs, and EG
6 NFs (
Figure 5b). Surface hydrophobicity of nanomaterials has been well documented to affect cellular association and uptake by phagocytic cells [
24,
30,
31,
32]. Surface hydrophobicity of nanomaterials facilitates interactions between nanomaterial surfaces and cellular membranes. This may lead to higher cell association of nanomaterials and occasionally increase the chance of recognition by particular receptors involved in cellular uptake. Our results show that cellular association of NFs decreased as the EG chain length increased (
Figure 5b), although these NFs commonly possess hydrophobic domains on their surface, as evidenced by the ANS fluorescence assay. These results suggest that longer EG chains inhibit hydrophobic interactions between the NF surface and cell membranes, which can be explained using the model structures presented in
Figure 9. The NF skeleton region, consisting mainly of hydrophobic amino acids, may facilitate the interaction with the cell membrane and the EG chain located on the lateral face of the NF may inhibit this interaction.
Results from CLSM observation revealed that EG
12 NFs were more efficiently internalized by JAWS cells than EG
6 NFs and EG
24 NFs (
Figure 6). Since the surface of EG
12 NFs is negatively charged, the mechanism for internalization of EG
12 NFs would be mainly via phagocytosis by scavenger receptor, which recognizes anion species, although further studies using some inhibitor for phagocytosis are required. Thus, the internalization behavior by non-phagocytic cells would be different from that by JAWS II cells. The internalization of EG
6 NFs was low, whereas their association propensity to cells was high. Because the size of nanomaterials can affect cell internalization [
48,
49,
50,
51], the dispersion state of NFs in aqueous media should be considered in addition to interactions between NFs and the cell surface. The results from DLS indicate that EG
12 and EG
24 NFs exist as isolated NFs without aggregation in aqueous media, whereas EG
6 NFs form large aggregates. This observation is consistent with CLSM images showing large aggregates adsorbed onto the cell surfaces. Thus, it is likely that the low efficiency of cellular internalization of EG
6 NFs can be attributed to their apparent size in water. The aggregation of EG
6 NFs is too large for cell uptake. This interpretation is consistent with a previous study that showed that cellular uptake of microparticles with a diameter of a few micrometers or more by phagocytic cells is slow and inefficient [
50,
51]. Thus, for development of NFs that are efficiently taken up by cells, it is important to design a EG chain length that allows modest interactions with cell membranes while ensuring water-dispersibility.
3.1.2. Cytotoxicity of NFs
Generally, nanomaterials with cationic or hydrophobic surfaces can induce significantly higher toxicities when compared with hydrophilic or anionic nanomaterials [
29]. A mechanism of cytotoxicity is cell membrane perturbation, including structural alternation, pore formation, and phase transitions, which cause nonspecific entrance of extracellular components to the cytosol. An increase in hydrophobic interactions between the surface of nanomaterials and cell membranes could perturb the membrane. In the present study, the cytotoxicity of EG
n NFs was found to increase in the order of EG
24, EG
12, and EG
6 (
Figure 7). These results indicate that longer EG chains inhibit the interaction between NFs and cell membranes, which leads to lower cytotoxicity of NFs with long EG chains. It is also possible that the cytotoxicity of NFs may be related to biological stress, e.g., induction of reactive oxygen species (ROS). The detailed mechanism of cytotoxicity by NFs is the subject of ongoing research.
3.1.3. DC Stimulation Ability of NFs
DC activation ability of EG
6 NFs and EG
12 NFs was much higher than that of EG
24 NFs. Matzinger and colleagues have proposed that hydrophobic portions in various biomolecules may be involved in the activation of the immune system [
22]. Hydrophobic components in molecules are usually masked from the external environment by hydrophilic components. However, when protein denaturation or cell disruption occur, these hydrophobic components become exposed and interact with particular surface receptors of immune cells, which activates the immune system. In agreement with the notion proposed by Matzinger, recently, the relationship between the surface hydrophobicity of nanomaterials and their immune stimulatory activities has been reported [
23,
24,
25,
26,
27,
28]. For example, Moyano and colleagues reported that the surface hydrophobicity of ligand-modified gold-nanoparticles was correlated with expression of pro-inflammatory cytokine genes in splenocytes from mice in vitro [
23]. Shima and colleagues also reported that the activation ability of nanoparticles was significantly affected by the hydrophobicity of polymers constituting the nanoparticles [
24]. In the present study, EG
6 NFs and EG
12 NFs stimulated DC maturation more effectively than EG
24 NFs, as evidenced by the quantitative evaluation of expressed co-stimulatory molecules (
Figure 8) and secreted immune-stimulatory cytokines, IL-6 and TNF-α (
Figure 9). Based on these results, it is reasonable to consider that the hydrophobic part of NFs plays an important role in DC activation. Longer EG chains seem to inhibit the recognition of hydrophobic surfaces of NFs by DC surface receptors in a similar manner to that described above. However the mechanisms responsible for DC maturation by EG
n NFs remain unclear and further studies are required. In addition, because IL-6 signaling cannot only promote anti-tumor-adaptive immunity, but also drive malignancy [
52], the role of IL-6 in this NFs-based vaccine system should be examined further in vivo.
3.2. Cellular Uptake, Toxicity, and DC Stimulatory Ability of Micelles
In the concentration range where EG
n peptides form micelle-like structures, their cellular association, cytotoxicity, and stimulation ability were not dependent on EG length (
Figure 5a,
Figure 7a, and
Figure 8). These results suggest that the surface components of the micelle-like structures would be almost the same. Poly(ethylene glycol) (PEG) interactions with biological components, including cellular membranes and proteins, are weak because of their nonionic hydrophilicity and high mobility [
53]. Thus, a low-level of interaction between the surface of the micelle-like structures and cell membrane components, including receptors involved in cellular uptake and DC maturation, led to lower uptake by DC, lower cytotoxicity, and no DC activation in comparison with NFs.
3.3. Cellular Uptake, Toxicity, and DC Stimulatory Ability of Monomeric Molecules
EG
n peptides exist as monomeric molecules in aqueous media at concentrations below ca. 15–30 μM. The cellular uptake of monomeric peptides was not dependent on EG length (
Figure 5a) and this may be attributed to size. Monomeric peptides are too small for efficient uptake by DC regardless to EG length. In addition, the monomeric peptides exhibited some cytotoxicity but no DC activation ability, which is in sharp contrast to the results with NFs (
Figure 7a,
Figure 8, and
Figure 9). These results suggest that monomeric peptides interact with cell membranes, possibly through an N-terminal hydrophobic region, but are not well recognized by receptors involved in DC activation. Recognition by receptors would be dependent on the size of the hydrophobic portion.
3.4. Design of NF-based Vaccines
Recently, various types of NFs for immunotherapy have been reported [
54,
55]. In particular, NFs formed from antigenic peptides conjugating to β-sheet-forming peptides have been recognized as very promising candidates for next-generation nanoparticle-based vaccines. In the present study, we demonstrated that the hydrophilic-hydrophobic balance of peptide NFs affects their cellular uptake, cytotoxicity, and DC activation ability. NFs consisting of EG with a moderate length (12-mer) showed the most balanced character: Highly efficient cell entry, low cytotoxicity, and high DC activation ability, indicating that the NFs have significant potential as NF-based vaccines, which can be used without additional adjuvants. In general, the relationship between toxicity and DC stimulation ability is a trade-off. It is important to improve the stimulation ability, while simultaneously reducing the cytotoxicity of the NFs. Our results demonstrate that such balance can be simply tuned by the length of the EG. This feature is important for designing safe NF-based vaccines with high immune stimulatory ability. In contrast to NF uptake, the uptake of micelles and monomeric peptides by DC cells inefficient and showed no DC stimulation ability independent of EG length. This result indicates that the assembly style of building block peptide molecules influences the properties of the nanoassembly formed from these building blocks. Finally, to develop NFs with strong immunity-inducing ability, it is necessary to precisely adjust the EG length and introduce intracellular environment-responsive links for efficient release of antigens in cells.
Although we focused on the effect of hydrophilic and hydrophobic balance of nanofibers on their interaction with cells, surface charge of nanofibers is also an important factor in determining the interaction. In general, positively charged nanomaterials are more effectively internalized to cells than negatively charged ones, but they are more toxic. Thus, for design of nanofiber vaccines, it is necessary to address the role of their surface charge. In addition to surface charge, the length of NFs is also an important factor determining their property as a nano-vaccine. In a previous study, we investigated the effect of nanofiber length on their cellular uptake using various NFs with different lengths (40 nm, 120 nm, 280 nm, 800 nm). The study demonstrated that nanofibers with a length of 280 nm were most effectively uptaken by phagocytic cells compared to the others (unpublished data). Based on this finding, we used NFs with a length of 230–260 nm for the cell experiments in the present study. However, other properties—e.g., cytotoxicity and the ability to stimulate immune cells etc.—could exhibit different size-dependencies. Therefore, the optimization of nanofiber length is also required for developing effective nanofiber vaccine.
The important attributes of a vaccine, which are antigen processing, antigen presentation, T-cell stimulation, and successful activation of adaptive immune response against target antigen, should also be evaluated. However, because the NFs used in this study comprised the minimum required block (β-sheet forming peptide, antigenic peptide, oligo(ethylene glycol)) and the antigen could not be released in the cells, the effective antigen presentation via MHC class I pathway and subsequent induction of immunity are not expected. Therefore, we are addressing the development of intracellular environment-responsive NFs for efficient release of antigens in cells and the characterization of their function to induce immunity in vivo.
4. Materials and Methods
4.1. Materials
21-amino-N-(9-fluorenylmethoxycarbonyl)-4,7,10,13,16,19-hexaoxaheneicosanoic acid (Fmoc-N-amido-dPEG6 acid), 39-amino-N-(9-fluorenylmethoxycarbonyl)-4, 7, 10, 13, 16, 19, 22, 25, 28, 31, 34, 37-dodecaoxanonatriacontanoic acid (Fmoc-N-amido-dPEG12 acid), and O-[N-(9-fluorenylmethoxycarbonyl)-2-aminoethyl]-O’-(2-carboxyethyl) undecaethyleneglycol (Fmoc-N-amido-dPEG24 acid) were purchased from Quanta BioDesign Ltd. (Plain City, OH, USA). 2-chlorotrityl chloride resin, N,N’-diisopropylethylamine (DIPEA), all the L-Fmoc amino acids, 1-[bis(dimethylamino)methylene]-1H-benzotriazolium 3-oxide hexafluorophosphate (HBTU), 1-hydroxybenzotriazole (HOBT), and piperidine were purchased from Watanabe Chemical Industries Ltd. (Hiroshima, Japan). N,N’-dimethylformamide (DMF), isopropanol, methanol, diethyl ether, hexafluoroisopropanol (HFIP), dichloromethane (CH2Cl2), trifluoroacetic acid (TFA), and granulocyte-macrophage colony-stimulating factor (GM-CSF) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Eagle’s minimal essential medium (EMEM), penicillin-streptomycin, and lipopolysaccharides from Escherichia coli O55:B5 were purchased from Sigma-Aldrich (St. Louis, MO, USA). N6-[(3′,6′-Dihydroxy-3-oxospiro[isobenzofuran-1(3H),9′-[9H]xanthen]-5-yl)carbonyl]-N2-[(9H-fluoren-9-ylmethoxy)carbonyl]-L-lysine (Fmoc-Lys(5-FAM)-OH) was purchased from AAT Bioquest (Sunnyvale, CA, USA). LysoTracker Red DND-99, and Hoechst 33342, trihydrochloride, trihydrate were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Fetal bovine serum (FBS) was purchased from Biowest (Nuaillé, France). Purified anti-mouse CD16/CD32 (2.4G2) and anti-mouse CD86 (B7-2) PE were purchased from Tonbo Biosciences (San Diego, CA, USA). The ELISA Kit for mouse TNF-alpha and IL-6 were purchased from R&D Systems (Minneapolis, MN, USA).
4.2. Experimental Methods
4.2.1. Synthesis of Building Block Molecules
Loading of resin: Fmoc-N-amido-dPEG12 was dehydrated by azeotropy with benzene prior to use. A solution of Fmoc-N-amido-dPEG12 acid (0.238 mmol) and DIPEA (0.952 mmol) in CH2Cl2 (2.6 mL) was added to 2-chlorotrityl chloride resin (0.397 mmol, 1.5 mmol/g loading max) for 12 h.
Peptide synthesis: Coupling reactions were performed using a standard Fmoc protocol. The coupling cycle included 3 repeats of Fmoc deprotection (20% piperidine in DMF, 5 min), a wash in DMF, two repeats of amino acid coupling: L-Fmoc amino acids (4 eq.), HBTU (3.6 eq.), HOBt (4 eq.), and DIPEA (8 eq.) for 30 min, and a final DMF wash. After all coupling reactions, the obtained peptides were cleaved from the resin using a solution of H2O/TFA/triisopropylsilane (100:5:2 volume ratio) containing 500 mM phenol for 2 h. The resulting peptides were precipitated in ice cold diethyl ether, filtered, centrifuged, and washed with diethyl ether. The crude peptides were purified by reversed-phase high-performance liquid chromatography (RP-HPLC, SPD-10A and LC-10A, Shimadzu Scientific Instruments, Kyoto, Japan). Other building blocks with different EG lengths were synthesized by a similar procedure. Molecular weight was analyzed by MALDI-TOF mass (autoflex speed system, Bruker, Billerica, MA, USA). MS (MALDI-TOF): EG6; Cald. MASS: 2234.92, Obsd. MASS: 2234.359, EG12; Cald. MASS: 2499.12, Obsd. MASS: 2498.11, EG24; Cald. MASS: 3027.52, Obsd. MASS: 3027.04.
Fluorescence-labeled building block peptides were synthesized using Fmoc-Lys(5-FAM)-OH as the first amino acid residue by a similar procedure. MS (MALDI-TOF): EG6-FAM; Cald. MASS: 2721.41, Obsd. MASS: 2720.84, EG12-FAM; Cald. MASS: 2985.61, Obsd. MASS: 2985.26, EG24-FAM; Cald. MASS: 3514.01, Obsd. MASS: 3514.65.
4.2.2. Preparation of Antigen-Loaded Peptide NFs
EGn peptide was dissolved in HFIP and dried with nitrogen flow to allow film formation. The obtained film was re-dissolved at a concentration of 30 mM in DMSO. The resulting solution (15 μL) was added to PBS (285 μL) to a final concentration of 1.5 mM and incubated at 60 °C for 24 h. Following incubation, the resulting peptide nanofiber dispersion was dialyzed against PBS for 24 h using dialysis membrane (MWCO 8,000, GE Healthcare, Chicago, IL, USA) to remove DMSO and free peptides. For cell-based experiments (cytotoxicity, DC maturation), the length of NFs was controlled by filtration using a syringe filter with a pore size of 0.45 μm (GE Healthcare).
4.2.3. Preparation of Fluorescence-Labeled Antigen-Loaded Peptide NFs
The HFIP-treated mixture of EGn and EGn-FAM peptides was dissolved in DMSO; then, 15 μL of the solution was added to 285 μL PBS to give final concentrations of 1.425 mM for EGn and 0.071 mM for EGn-FAM. The solution was incubated at 60 °C for 24 h, and then the resulting peptide nanofiber dispersion was dialyzed against PBS for 24 h using dialysis membrane (MWCO 8,000, GE Healthcare) to remove DMSO and free peptides. The length of NFs was controlled by filtration using a syringe filter with a pore size of 0.45 μm (GE Healthcare).
4.2.4. Determination of Critical Aggregation Concentration
CAC was determined using the pyrene 1:3 method [
56]. First, a saturated solution of pyrene was prepared by mixing an excess of pyrene with PBS, and using the supernatant to dissolve EG
n peptides at a concentration of 150 mM (the stock solution). A concentration range of EG
n peptides from 2.5 μM to 100 μM was then prepared using serial dilutions of the stock solution with the saturated solution of pyrene. The final concentration of pyrene was equal in each solution. The fluorescence emission of pyrene was monitored using a fluorescence spectrometer (RF5300 PC, Shimadzu Scientific Instruments, Kyoto, Japan) with an excitation wavelength of 335 nm at 37 °C. The ratio of the emission intensities at 376 nm and 392 nm were then plotted as a function of the EG
n peptide concentration (log scale). The CAC was determined from an abrupt change in the slope of the plot using the least-squares fitting technique.
4.2.5. ThT Assay
PBS containing 10 μM Thioflavin T (ThT) was dispensed into a 96-well plate. EGn peptides solution (6 mM) was prepared and added to the 96-well plate, giving a final concentration of 300 μM. ThT fluorescence intensities at 480 nm (excitation; 440 nm) were monitored using a Genios microplate reader (TECAN, Männedorf, Switzerland) at 37 °C.
4.2.6. Measurement of Surface Hydrophobicity
The surface hydrophobicity of NFs in the solution was determined using an ANS fluorescent probe as previously reported [
41,
42]. A concentration range of EG
n NFs in PBS from 9.4 μM to 200 μM was prepared. The nanofiber dispersion was mixed with the equivalent volume of PBS containing 20 μM ANS. The intensities of ANS fluorescence ranging from 400 nm to 600 nm (excitation; 370 nm) were monitored using a fluorescence spectrometer (RF5300 PC) at 37 °C.
4.2.7. Cell Culture
JAWS II, a DC line derived from mouse bone marrow, was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were grown in EMEM supplemented with 20% FBS, 5 ng/mL murine GM-CSF, and antibiotics at 37 °C, 5% CO2.
4.2.8. Evaluation of Cellular Association of Peptide NFs
JAWS II cells were seeded into 12-well plates (2.5 × 10
5 per well) and cultured for 12 h at 37 °C in a humidified atmosphere (5% CO
2). After 12 h, the cells were washed with PBS and serum-free culture medium. The fluorescence-labeled peptide NF dispersion was added gently to the cells followed by incubation for 2 h at 37 °C. Following incubation, the cells were washed with PBS and 0.2% trypan blue aqueous solution, which was used to quench the flourescence from surface-adsorbed NFs [
57]. The cells were then detached using trypsin and subsequently analyzed by FCM (Guava EasyCyte Plus, Millipore, Burlington, MA, USA). As a comparison to peptide NFs, the cellular uptake of the building block peptides without heat treatment was investigated under the same conditions.
4.2.9. CLSM Observation of NF-Treated Cells
JAWS II cells (1.5 × 105) were cultured for 12 h in a 35 mm glass-bottom dish and subsequently washed with PBS and serum-free culture medium. Fluorescein-labeled peptide NFs were gently added to the cells, followed by incubation for 2 h at 37 °C with 5% CO2. After incubation, the cells were washed with PBS, and then incubated for 5 min with a solution containing LysoTracker Red DND-99 (50 nM) and Hoechst 33342, trihydrochloride, trihydrate (3.24 μM). LysoTracker Red DND-99 and Hoechst 33342 were used to stain the intracellular acidic compartments and nuclei, respectively. After staining, the cells were washed twice with PBS, then observed by CLSM using an FV10i microscope (Olympus, Tokyo, Japan).
4.2.10. Quantitative Expression Analysis of Co-Stimulatory Molecules and Cytokines from NF-Treated Cells
The expression of co-stimulatory molecules and cytokines was evaluated by specific immunostaining, as well as by ELISA. For immunostaining, JAWS II cells (2 × 105) were cultured for 12 h in a 24-well plate followed by washing with PBS containing 3% FBS and 0.05% NaN3, and then with serum-free culture medium. The DCs were pulsed with peptide NFs for 24 h, and then immunostained with a mouse monoclonal antibody for CD86 (a maturation marker), and subsequently analyzed by FCM to estimate their CD86 expression level. For quantitative analysis of TNF-α and IL-6 expression, the supernatants following the 24 h co-incubation of DCs with peptide NFs were collected and analyzed using an ELISA kit. The maturation of JAWS II cells cultured in medium with and without LPS (1 μg/mL) was evaluated as the positive and negative control, respectively. In addition, to compare the DC-activation ability between the NFs and heat-untreated building block peptides, JAWS II cells cultured with the peptides were also evaluated.
4.2.11. Evaluation of Cytotoxicity of Peptide NFs
The cytotoxicity of peptide NFs was evaluated using a Cell Counting Kit-8 (Dojindo Molecular Technologies, Kumamoto, Japan) according to the manufacturer’s instructions. Briefly, JAWS II cells were seeded into 96-well plates (1.0 × 10
5 per well) and cultured for 12 h at 37 °C in a humidified atmosphere (5% CO
2). After 12 h, the cells were washed with PBS and serum-free culture medium. The nanofiber dispersion was gently added to the cells followed by incubation for 24 h. The cells were washed with PBS three times and the medium was replaced with a solution containing 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8), and 1-methoxy-5-methylphenazinium methylsulfate at a 10-fold dilution. After a 2 h incubation, the absorbance was measured at 420 nm using a plate reader (Multiskan JX, Thermo Fisher Scientific, Waltham, MA, USA). The relative cellular activity was calculated using the following equation:
where
A420 nm is the absorbance at 420 nm,
A420 nm (untreated cells) is the absorbance at 420 nm after incubation in the absence of peptide NFs, and
A420 nm (blank) is the absorbance of medium containing WST-8 reagent at 420 nm. As a comparison, the cytotoxicity of building block peptides without heat treatment was investigated in a similar manner.
4.3. Other Characterizations
TEM measurements were performed using a JEM-1200EX II (JEOL, Tokyo, Japan) with an acceleration voltage of 85 keV. The samples were negatively stained with 0.1% phosphotungstate. p-potentials of NFs were measured using a Micro-Electrophoresis Zeta Potential Analyzer Model 502 (Nihon Rufuto, Tokyo, Japan). DLS analysis was performed using a particle size analyzer (ELSZ-1000, Otsuka Electronics, Osaka, Japan) at 25 °C. The light source was a He-Ne laser (630 nm) set at an 1ngle of 45°. Experimental data were analyzed using the marquardt provided by the manufacturer. CD spectra were measured using a J-720 spectropolarimeter (Jasco, Tokyo, Japan) at 25 °C. The data were obtained using a 0.1 cm path length cell at a scan speed of 20 nm/min.
5. Conclusions
This study showed that the hydrophilic-hydrophobic balance of antigen-loaded NFs significantly impacted on their cellular uptake, cytotoxicity, and DC stimulation ability, which differs noticeably from the results observed for micelles formed from the same components of NFs. Building blocks consisting of β-sheet-forming peptides conjugated with antigenic peptides and hydrophilic EG with different lengths (6-mer, 12-mer and 24-mer) were found to successfully form NFs with homogenous widths. The uptake of NFs consisting of EG with a moderate length (12-mer) by DC was effective, and these NFs activated DC without exhibiting significant cytotoxicity. Increasing the EG chain length significantly reduced the interactions with cells. Conversely, decreasing the EG chain length enhanced DC activation ability but increased toxicity and impaired water-dispersibility, resulting in low cellular uptake. Thus, since cell entry, cytotoxicity, and the immune stimulation ability of antigen-loaded NFs can be tuned by the length of the EG moiety, the antigen-loaded NFs have potential as NF-based vaccines that can be used without additional adjuvants. In order to achieve efficient immune response in vivo, the development of intracellular environment-responsive NFs is now in progress. We believe the findings obtained in this study contribute to the understanding of the interaction between the surface of one-dimensional assemblies and cells, and provide useful design guidelines for development of effective NF-based vaccines.