Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions
Abstract
:1. What Are Pheromones?
2. Structural Diversity of Ascaroside (ascr) Pheromones
2.1. Daumone, the First Chemically Characterized Ascr Pheromone
2.2. Identification of Diverse Ascr Pheromones in Nematodes
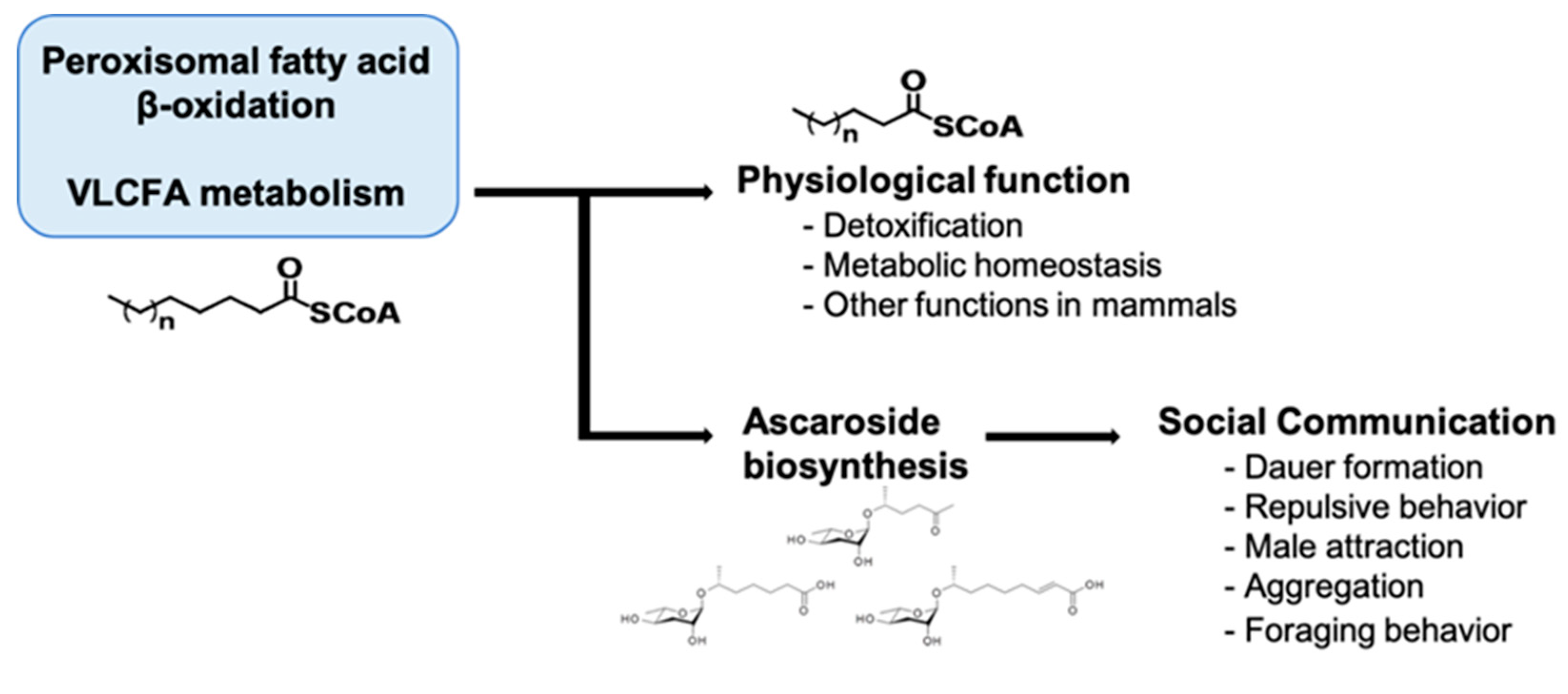
3. Ascr Pheromone Biosynthesis and Metabolic Regulation
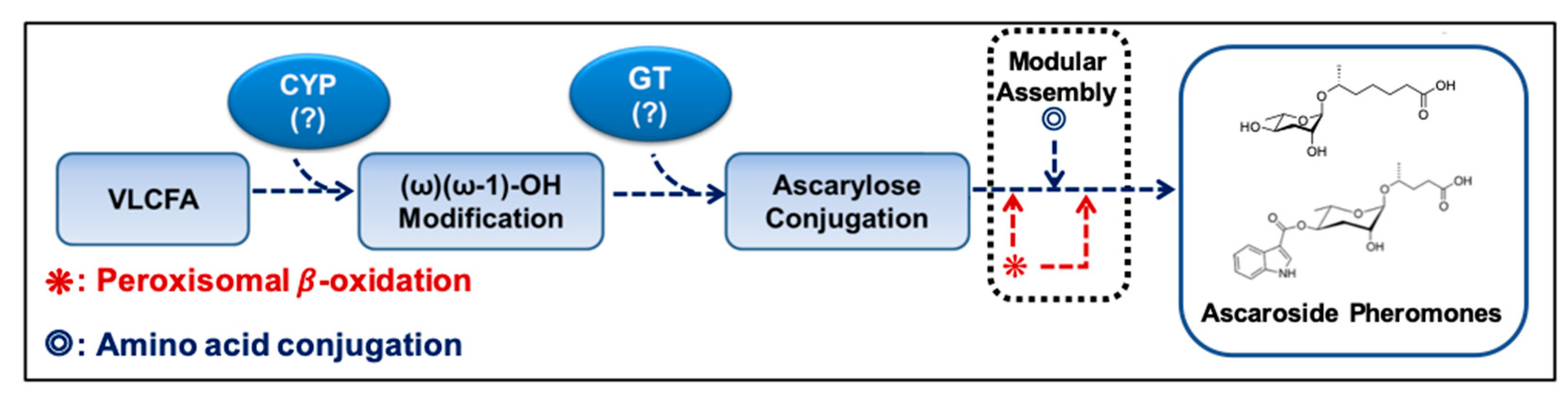
3.1. Ascr Pheromone Biosynthesis
3.2. Transcriptional Regulation of Ascr Pheromone Biosynthesis by Environmental Stressors
4. Pleiotropic Neuronal Functions of Ascr Pheromones
4.1. Roles of Ascr Pheromones in Development and Aging
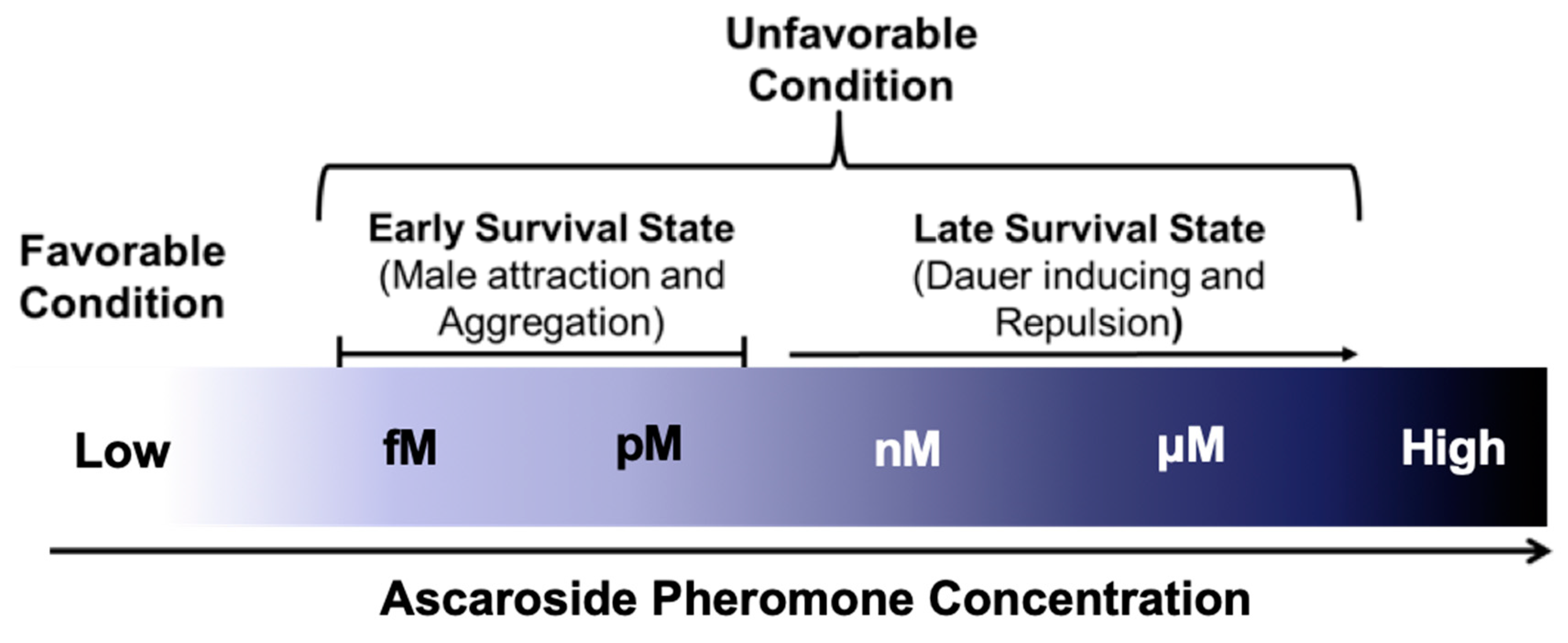
4.2. Neuronal Effects of Ascr Pheromones on Nematode Social Behaviors
5. Implications of Ascr Pheromone Metabolism in Neuroprotection
5.1. Implications of Ascr Pheromone Biosynthesis Gene Deficiencies in Neuronal Disorders
5.2. Implications of Ascr Pheromone Signaling in Chemotactic Responses
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Karlson, P.; Lüscher, M. ‘Pheromones’: A New Term for a Class of Biologically Active Substances. Nature 1959, 183, 55. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.D. Pheromones. Curr. Biol. 2017, 27, R739–R743. [Google Scholar] [CrossRef] [PubMed]
- Brennan, P.A.; Zufall, F. Pheromonal communication in vertebrates. Nature 2006, 444, 308. [Google Scholar] [CrossRef] [PubMed]
- Tillman, J.A.; Seybold, S.J.; Jurenka, R.A.; Blomquist, G.J. Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 1999, 29, 481–514. [Google Scholar] [CrossRef]
- Abel, E.L. Alarm substance emitted by rats in the forced-swim test is a low volatile pheromone. Physiol. Behav. 1991, 50, 723–727. [Google Scholar] [CrossRef]
- McGlone, J.J.; Anderson, D.L. Synthetic maternal pheromone stimulates feeding behavior and weight gain in weaned pigs. J. Anim. Sci. 2002, 80, 3179–3183. [Google Scholar] [CrossRef] [PubMed]
- Wyatt Tristram, D. The search for human pheromones: The lost decades and the necessity of returning to first principles. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142994. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.J.; Preti, G. Facts, fallacies, fears, and frustrations with human pheromones. Anat. Rec. A. Discov. Mol. Cell. Evol. Biol. 2004, 281A, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- McGann, J.P. Poor human olfaction is a 19th-century myth. Science 2017, 356, eaam7263. [Google Scholar] [CrossRef]
- Meredith, M. Human Vomeronasal Organ Function: A Critical Review of Best and Worst Cases. Chem. Senses 2001, 26, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Cassada, R.C.; Russell, R.L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 1975, 46, 326–342. [Google Scholar] [CrossRef]
- Golden, J.W.; Riddle, D.L. A pheromone influences larval development in the nematode Caenorhabditis elegans. Science 1982, 218, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Jeong, P.-Y.; Jung, M.; Yim, Y.-H.; Kim, H.; Park, M.; Hong, E.; Lee, W.; Kim, Y.H.; Kim, K.; Paik, Y.-K. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature 2005, 433, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Viney, M.E.; Franks, N.R. Is dauer pheromone of Caenorhabditis elegans really a pheromone? Naturwissenschaften 2004, 91, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Edison, A.S. Caenorhabditis elegans pheromones regulate multiple complex behaviors. Curr. Opin. Neurobiol. 2009, 19, 378–388. [Google Scholar] [CrossRef] [Green Version]
- von Reuss, S.H.; Bose, N.; Srinivasan, J.; Yim, J.J.; Judkins, J.C.; Sternberg, P.W.; Schroeder, F.C. Comparative Metabolomics Reveals Biogenesis of Ascarosides, a Modular Library of Small-Molecule Signals in C. elegans. J. Am. Chem. Soc. 2012, 134, 1817–1824. [Google Scholar] [CrossRef]
- von Reuss, S.H.; Dolke, F.; Dong, C. Ascaroside Profiling of Caenorhabditis elegans Using Gas Chromatography–Electron Ionization Mass Spectrometry. Anal. Chem. 2017, 89, 10570–10577. [Google Scholar] [CrossRef]
- Fauré-Frémiet, E. Le Cycle Erminatif Chez l’Ascaris Megalocephala; Masson: Issy les Moulineaux, France, 1913. [Google Scholar]
- Flury, F. Zur Chemie und Toxikologie der Ascariden. Arch. Für Exp. Pathol. Pharmakol. 1912, 67, 275–392. [Google Scholar] [CrossRef] [Green Version]
- Fouquey, C.; Polonsky, J.; Lederer, E. Chemical structure of ascarylic alcohol isolated from Parascaris equorum. Bull. Soc. Chim. Biol. 1957, 39, 101–132. [Google Scholar]
- Bartley, J.P.; Bennett, E.A.; Darben, P.A. Structure of the Ascarosides from Ascaris suum. J. Nat. Prod. 1996, 59, 921–926. [Google Scholar] [CrossRef]
- Butcher, R.A.; Fujita, M.; Schroeder, F.C.; Clardy, J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat. Chem. Biol. 2007, 3, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A.; Ragains, J.R.; Kim, E.; Clardy, J. A potent dauer pheromone component in Caenorhabditis elegans that acts synergistically with other components. Proc. Natl. Acad. Sci. USA 2008, 105, 14288–14292. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A.; Ragains, J.R.; Clardy, J. An Indole-Containing Dauer Pheromone Component with Unusual Dauer Inhibitory Activity at Higher Concentrations. Org. Lett. 2009, 11, 3100–3103. [Google Scholar] [CrossRef] [PubMed]
- Pungaliya, C.; Srinivasan, J.; Fox, B.W.; Malik, R.U.; Ludewig, A.H.; Sternberg, P.W.; Schroeder, F.C. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 7708–7713. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, J.; Kaplan, F.; Ajredini, R.; Zachariah, C.; Alborn, H.T.; Teal, P.E.A.; Malik, R.U.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature 2008, 454, 1115–1118. [Google Scholar] [CrossRef]
- Srinivasan, J.; von Reuss, S.H.; Bose, N.; Zaslaver, A.; Mahanti, P.; Ho, M.C.; O’Doherty, O.G.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A Modular Library of Small Molecule Signals Regulates Social Behaviors in Caenorhabditis elegans. PLOS Biol. 2012, 10, e1001237. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A.; Ragains, J.R.; Li, W.; Ruvkun, G.; Clardy, J.; Mak, H.Y. Biosynthesis of the Caenorhabditis elegans dauer pheromone. Proc. Natl. Acad. Sci. USA 2009, 106, 1875–1879. [Google Scholar] [CrossRef]
- Joo, H.-J.; Yim, Y.-H.; Jeong, P.-Y.; Jin, Y.-X.; Lee, J.-E.; Kim, H.; Jeong, S.-K.; Chitwood, D.J.; Paik, Y.-K. Caenorhabditis elegans utilizes dauer pheromone biosynthesis to dispose of toxic peroxisomal fatty acids for cellular homoeostasis. Biochem. J. 2009, 422, 61–71. [Google Scholar] [CrossRef]
- Joo, H.-J.; Kim, K.-Y.; Yim, Y.-H.; Jin, Y.-X.; Kim, H.; Kim, M.-Y.; Paik, Y.-K. Contribution of the peroxisomal acox gene to the dynamic balance of daumone production in Caenorhabditis elegans. J. Biol. Chem. 2010, 285, 29319–29325. [Google Scholar] [CrossRef]
- Joo, H.-J.; Park, S.; Kim, K.-Y.; Kim, M.-Y.; Kim, H.; Park, D.; Paik, Y.-K. HSF-1 is involved in regulation of ascaroside pheromone biosynthesis by heat stress in Caenorhabditis elegans. Biochem. J. 2016, 473, 789–796. [Google Scholar] [CrossRef]
- Panda, O.; Akagi, A.E.; Artyukhin, A.B.; Judkins, J.C.; Le, H.H.; Mahanti, P.; Cohen, S.M.; Sternberg, P.W.; Schroeder, F.C. Biosynthesis of Modular Ascarosides in C. elegans. Angew. Chem. Int. Ed. 2017, 56, 4729–4733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Feng, L.; Chinta, S.; Singh, P.; Wang, Y.; Nunnery, J.K.; Butcher, R.A. Acyl-CoA oxidase complexes control the chemical message produced by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2015, 112, 3955–3960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, K.; Jones, R.A.; Bruner, S.D.; Butcher, R.A. Structural characterization of acyl-CoA oxidases reveals a direct link between pheromone biosynthesis and metabolic state in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2016, 113, 10055–10060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Perez, D.H.; Jones Lipinski, R.A.; Butcher, R.A. Acyl-CoA Oxidases Fine-Tune the Production of Ascaroside Pheromones with Specific Side Chain Lengths. ACS Chem. Biol. 2018, 13, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, Y.; Zhang, X.; Bhar, S.; Jones Lipinski, R.A.; Han, J.; Feng, L.; Butcher, R.A. Biosynthetic tailoring of existing ascaroside pheromones alters their biological function in C. elegans. eLife 2018, 7, e33286. [Google Scholar] [CrossRef]
- Bose, N.; Ogawa, A.; von Reuss, S.H.; Yim, J.J.; Ragsdale, E.J.; Sommer, R.J.; Schroeder, F.C. Complex Small-Molecule Architectures Regulate Phenotypic Plasticity in a Nematode. Angew. Chem. Int. Ed. 2012, 51, 12438–12443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bose, N.; Meyer, J.M.; Yim, J.J.; Mayer, M.G.; Markov, G.V.; Ogawa, A.; Schroeder, F.C.; Sommer, R.J. Natural Variation in Dauer Pheromone Production and Sensing Supports Intraspecific Competition in Nematodes. Curr. Biol. 2014, 24, 1536–1541. [Google Scholar] [CrossRef] [Green Version]
- Choe, A.; von Reuss, S.H.; Kogan, D.; Gasser, R.B.; Platzer, E.G.; Schroeder, F.C.; Sternberg, P.W. Ascaroside Signaling Is Widely Conserved among Nematodes. Curr. Biol. 2012, 22, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Choe, A.; Chuman, T.; von Reuss, S.H.; Dossey, A.T.; Yim, J.J.; Ajredini, R.; Kolawa, A.A.; Kaplan, F.; Alborn, H.T.; Teal, P.E.A.; et al. Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proc. Natl. Acad. Sci. USA 2012, 109, 20949–20954. [Google Scholar] [CrossRef]
- Dong, C.; Dolke, F.; Reuss, S.H. von Selective MS screening reveals a sex pheromone in Caenorhabditis briggsae and species-specificity in indole ascaroside signalling. Org. Biomol. Chem. 2016, 14, 7217–7225. [Google Scholar] [CrossRef]
- Dong, C.; Reilly, D.K.; Bergame, C.; Dolke, F.; Srinivasan, J.; von Reuss, S.H. Comparative Ascaroside Profiling of Caenorhabditis Exometabolomes Reveals Species-Specific (ω) and (ω–2)-Hydroxylation Downstream of Peroxisomal β-Oxidation. J. Org. Chem. 2018, 83, 7109–7120. [Google Scholar] [CrossRef] [PubMed]
- Izrayelit, Y.; Robinette, S.L.; Bose, N.; von Reuss, S.H.; Schroeder, F.C. 2D NMR-Based Metabolomics Uncovers Interactions between Conserved Biochemical Pathways in the Model Organism Caenorhabditis elegans. ACS Chem. Biol. 2013, 8, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Zagoriy, V.; Matyash, V.; Kurzchalia, T. Long-Chain O-Ascarosyl-alkanediols Are Constitutive Components of Caenorhabditis elegans but Do Not Induce Dauer Larva Formation. Chem. Biodivers. 2010, 7, 2016–2022. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.J.; Davies, D.B. Type-specific carbohydrate antigens of pathogenic bacteria. Part 1: Enterobacteriaceae. Carbohydr. Polym. 1991, 14, 241–279. [Google Scholar] [CrossRef]
- Thorson, J.S.; Lo, S.F.; Liu, H.W.; Hutchinson, C.R. Biosynthesis of 3, 6-dideoxyhexoses: New mechanistic reflections upon 2, 6-dideoxy, 4, 6-dideoxy, and amino sugar construction. J. Am. Chem. Soc. 1993, 115, 6993–6994. [Google Scholar] [CrossRef]
- Thorson, J.S.; Lo, S.F.; Ploux, O.; He, X.; Liu, H.W. Studies of the biosynthesis of 3,6-dideoxyhexoses: Molecular cloning and characterization of the asc (ascarylose) region from Yersinia pseudotuberculosis serogroup VA. J. Bacteriol. 1994, 176, 5483–5493. [Google Scholar] [CrossRef]
- Trefzer, A.; Bechthold, A.; Salas, J.A. Genes and enzymes involved in deoxysugar biosynthesis in bacteria. Nat. Prod. Rep. 1999, 16, 283–299. [Google Scholar] [CrossRef]
- Olson, S.K.; Greenan, G.; Desai, A.; Müller-Reichert, T.; Oegema, K. Hierarchical assembly of the eggshell and permeability barrier in C. elegans. J. Cell Biol 2012, 198, 731–748. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.A.; Davies, G.J.; Bulone, V.; Henrissat, B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem. J. 1997, 326, 929–939. [Google Scholar] [CrossRef]
- Koeller, K.M.; Wong, C.-H. Synthesis of complex carbohydrates and glycoconjugates: Enzyme-based and programmable one-pot strategies. Chem. Rev. 2000, 100, 4465–4494. [Google Scholar] [CrossRef]
- Gems, D.; McElwee, J.J. Broad spectrum detoxification: The major longevity assurance process regulated by insulin/IGF-1 signaling? Mech. Ageing Dev. 2005, 126, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, T.H.; Dodd, A.K. Xenobiotic detoxification in the nematode Caenorhabditis elegans. J. Exp. Zoolog. A Comp. Exp. Biol. 2006, 305A, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Tukey, R.H.; Strassburg, C.P. Human UDP-Glucuronosyltransferases: Metabolism, Expression, and Disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616. [Google Scholar] [CrossRef] [PubMed]
- Artyukhin, A.B.; Yim, J.J.; Srinivasan, J.; Izrayelit, Y.; Bose, N.; von Reuss, S.H.; Jo, Y.; Jordan, J.M.; Baugh, L.R.; Cheong, M.; et al. Succinylated Octopamine Ascarosides and a New Pathway of Biogenic Amine Metabolism in Caenorhabditis elegans. J. Biol. Chem. 2013, 288, 18778–18783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motley, A.M.; Hettema, E.H.; Ketting, R.; Plasterk, R.; Tabak, H.F. Caenorhabditis elegans has a single pathway to target matrix proteins to peroxisomes. EMBO Rep. 2000, 1, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Togo, S.H.; Maebuchi, M.; Bun-ya, M.; Haraguchi, C.M.; Kamiryo, T. Peroxisomes of the nematode Caenorhabditis elegans: Distribution and morphological characteristics. Histochem. Cell Biol. 2002, 118, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Von Reuss, S.H. Exploring Modular Glycolipids Involved in Nematode Chemical Communication. CHIMIA Int. J. Chem. 2018, 72, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-Y.; Joo, H.-J.; Kwon, H.-W.; Kim, H.; Hancock, W.S.; Paik, Y.-K. Development of a method to quantitate nematode pheromone for study of small-molecule metabolism in Caenorhabditis elegans. Anal. Chem. 2013, 85, 2681–2688. [Google Scholar] [CrossRef]
- Kaplan, F.; Srinivasan, J.; Mahanti, P.; Ajredini, R.; Durak, O.; Nimalendran, R.; Sternberg, P.W.; Teal, P.E.A.; Schroeder, F.C.; Edison, A.S.; et al. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PloS ONE 2011, 6, e17804. [Google Scholar] [CrossRef]
- Greene, J.S.; Brown, M.; Dobosiewicz, M.; Ishida, I.G.; Macosko, E.Z.; Zhang, X.; Butcher, R.A.; Cline, D.J.; McGrath, P.T.; Bargmann, C.I. Balancing selection shapes density-dependent foraging behaviour. Nature 2016, 539, 254–258. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 2009, 458, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Jeong, P.-Y.; Kwon, M.-S.; Joo, H.-J.; Paik, Y.-K. Molecular Time-Course and the Metabolic Basis of Entry into Dauer in Caenorhabditis elegans. PLOS ONE 2009, 4, e4162. [Google Scholar] [CrossRef]
- Jones, S.J.M.; Riddle, D.L.; Pouzyrev, A.T.; Velculescu, V.E.; Hillier, L.; Eddy, S.R.; Stricklin, S.L.; Baillie, D.L.; Waterston, R.; Marra, M.A. Changes in Gene Expression Associated with Developmental Arrest and Longevity in Caenorhabditis elegans. Genome Res. 2001, 11, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, W.G.; Riddle, D.L. Developmental regulation of energy metabolism in Caenorhabditis elegans. Dev. Biol. 1989, 132, 167–173. [Google Scholar] [CrossRef]
- Wang, J.; Kim, S.K. Global analysis of dauer gene expression in Caenorhabditis elegans. Development 2003, 130, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, M.; Lee, D.; Kim, H.; Hwang, H.; Kim, H.; Park, S.; Paik, Y.; Lee, J. Nictation, a dispersal behavior of the nematode Caenorhabditis elegans, is regulated by IL2 neurons. Nat. Neurosci. 2012, 15, 107–112. [Google Scholar] [CrossRef]
- Lee, D.; Yang, H.; Kim, J.; Brady, S.; Zdraljevic, S.; Zamanian, M.; Kim, H.; Paik, Y.; Kruglyak, L.; Andersen, E.C.; et al. The genetic basis of natural variation in a phoretic behavior. Nat. Commun. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Sato, K.; Shibuya, M.; Zeiger, D.M.; Butcher, R.A.; Ragains, J.R.; Clardy, J.; Touhara, K.; Sengupta, P. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science 2009, 326, 994–998. [Google Scholar] [CrossRef]
- Sommer, R.J.; Ogawa, A. Hormone Signaling and Phenotypic Plasticity in Nematode Development and Evolution. Curr. Biol. 2011, 21, R758–R766. [Google Scholar] [CrossRef] [Green Version]
- McGrath, P.T.; Xu, Y.; Ailion, M.; Garrison, J.L.; Butcher, R.A.; Bargmann, C.I. Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 2011, 477, 321–325. [Google Scholar] [CrossRef]
- Park, D.; O’Doherty, I.; Somvanshi, R.K.; Bethke, A.; Schroeder, F.C.; Kumar, U.; Riddle, D.L. Interaction of structure-specific and promiscuous G-protein-coupled receptors mediates small-molecule signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2012, 109, 9917–9922. [Google Scholar] [CrossRef]
- Neal, S.J.; Park, J.; DiTirro, D.; Yoon, J.; Shibuya, M.; Choi, W.; Schroeder, F.C.; Butcher, R.A.; Kim, K.; Sengupta, P. A Forward Genetic Screen for Molecules Involved in Pheromone-Induced Dauer Formation in Caenorhabditis elegans. G3 Genes Genomes Genet. 2016, 6, 1475–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Choi, W.; Dar, A.R.; Butcher, R.A.; Kim, K. Neuropeptide Signaling Regulates Pheromone-Mediated Gene Expression of a Chemoreceptor Gene in C. elegans. Mol. Cells 2018, 42, 28–35. [Google Scholar] [PubMed]
- Ludewig, A.H.; Izrayelit, Y.; Park, D.; Malik, R.U.; Zimmermann, A.; Mahanti, P.; Fox, B.W.; Bethke, A.; Doering, F.; Riddle, D.L.; et al. Pheromone sensing regulates Caenorhabditis elegans lifespan and stress resistance via the deacetylase SIR-2.1. Proc. Natl. Acad. Sci. USA 2013, 110, 5522–5527. [Google Scholar] [CrossRef] [PubMed]
- Narayan, A.; Venkatachalam, V.; Durak, O.; Reilly, D.K.; Bose, N.; Schroeder, F.C.; Samuel, A.D.T.; Srinivasan, J.; Sternberg, P.W. Contrasting responses within a single neuron class enable sex-specific attraction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2016, 113, E1392–E1401. [Google Scholar] [CrossRef] [PubMed]
- Fagan, K.A.; Luo, J.; Lagoy, R.C.; Schroeder, F.C.; Albrecht, D.R.; Portman, D.S. A Single-Neuron Chemosensory Switch Determines the Valence of a Sexually Dimorphic Sensory Behavior. Curr. Biol. 2018, 28, 902–914. [Google Scholar] [CrossRef]
- Park, D.; Hahm, J.-H.; Park, S.; Ha, G.; Chang, G.-E.; Jeong, H.; Kim, H.; Kim, S.; Cheong, E.; Paik, Y.-K. A conserved neuronal DAF-16/FoxO plays an important role in conveying pheromone signals to elicit repulsion behavior in Caenorhabditis elegans. Sci. Rep. 2017, 7, 7260. [Google Scholar] [CrossRef]
- Hong, M.; Ryu, L.; Ow, M.C.; Kim, J.; Je, A.R.; Chinta, S.; Huh, Y.H.; Lee, K.J.; Butcher, R.A.; Choi, H.; et al. Early Pheromone Experience Modifies a Synaptic Activity to Influence Adult Pheromone Responses of C. elegans. Curr. Biol. 2017, 27, 3168–3177. [Google Scholar] [CrossRef]
- Ryu, L.; Cheon, Y.; Huh, Y.H.; Pyo, S.; Chinta, S.; Choi, H.; Butcher, R.A.; Kim, K. Feeding state regulates pheromone-mediated avoidance behavior via the insulin signaling pathway in Caenorhabditis elegans. EMBO J. 2018, 37, e98402. [Google Scholar] [CrossRef]
- Zhang, Y.K.; Sanchez-Ayala, M.A.; Sternberg, P.W.; Srinivasan, J.; Schroeder, F.C. Improved Synthesis for Modular Ascarosides Uncovers Biological Activity. Org. Lett. 2017, 19, 2837–2840. [Google Scholar] [CrossRef]
- Fenk, L.A.; Bono, M. de Memory of recent oxygen experience switches pheromone valence in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2017, 114, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.S.; Dobosiewicz, M.; Butcher, R.A.; McGrath, P.T.; Bargmann, C.I. Regulatory changes in two chemoreceptor genes contribute to a Caenorhabditis elegans QTL for foraging behavior. eLife 2016, 5, e21454. [Google Scholar] [CrossRef] [PubMed]
- Bell, G. The Masterpiece of Nature: The Evolution and Genetics of Sexuality; CUP Archive: Cambridge, UK, 1982. [Google Scholar]
- Dacks, J.; Roger, A.J. The First Sexual Lineage and the Relevance of Facultative Sex. J. Mol. Evol. 1999, 48, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Dubnau, D. Genetic competence in Bacillus subtilis. Microbiol. Mol. Biol. Rev. 1991, 55, 395–424. [Google Scholar]
- Gemmill, A.W.; Viney, M.E.; Read, A.F. Host Immune Status Determines Sexuality in a Parasitic Nematode. Evolution 1997, 51, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.H.; Stern, D.B.; Witman, G.B. The Chlamydomonas Sourcebook; Elsevier: San Diego, CA, USA, 2009. [Google Scholar]
- Kleiven, O.T.; Larsson, P.; Hobæk, A. Sexual reproduction in Daphnia magna requires three stimuli. Oikos 1992, 197–206. [Google Scholar] [CrossRef]
- Lynch, M.; Bürger, R.; Butcher, D.; Gabriel, W. The Mutational Meltdown in Asexual Populations. J. Hered. 1993, 84, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, B.; Breeden, L. CLN1 and Its Repression by Xbp1 Are Important for Efficient Sporulation in Budding Yeast. Mol. Cell. Biol. 2000, 20, 478–487. [Google Scholar] [CrossRef]
- Muller, H.J. The relation of recombination to mutational advance. Mutat. Res. Mol. Mech. Mutagen. 1964, 1, 2–9. [Google Scholar] [CrossRef]
- Morran, L.T.; Cappy, B.J.; Anderson, J.L.; Phillips, P.C. Sexual Partners for the Stressed: Facultative Outcrossing in the Self-Fertilizing Nematode Caenorhabditis Elegans. Evolution 2009, 63, 1473–1482. [Google Scholar] [CrossRef]
- Wharam, B.; Weldon, L.; Viney, M. Pheromone modulates two phenotypically plastic traits—adult reproduction and larval diapause—In the nematode Caenorhabditis elegans. BMC Evol. Biol. 2017, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Izrayelit, Y.; Srinivasan, J.; Campbell, S.L.; Jo, Y.; von Reuss, S.H.; Genoff, M.C.; Sternberg, P.W.; Schroeder, F.C. Targeted Metabolomics Reveals a Male Pheromone and Sex-Specific Ascaroside Biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 2012, 7, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Aprison, E.Z.; Ruvinsky, I. Sex Pheromones of C. elegans Males Prime the Female Reproductive System and Ameliorate the Effects of Heat Stress. PLOS Genet. 2015, 11, e1005729. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Runnels, A.M.; Murphy, C.T. Mating and male pheromone kill Caenorhabditis males through distinct mechanisms. eLife. 2017, 6, e23493. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Blumenthal, T.; Meyer, B.J.; Priess, J.R. C. elegans II, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Berger, J.; Dorninger, F.; Forss-Petter, S.; Kunze, M. Peroxisomes in brain development and function. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2016, 1863, 934–955. [Google Scholar] [CrossRef]
- Clayton, P.T. Clinical consequences of defects in peroxisomal β-oxidation. Biochem. Soc. Trans. 2001, 29, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Crane, D.I. Revisiting the neuropathogenesis of Zellweger syndrome. Neurochem. Int. 2014, 69, 1–8. [Google Scholar] [CrossRef]
- De Munter, S.; Verheijden, S.; Régal, L.; Baes, M. Peroxisomal Disorders: A Review on Cerebellar Pathologies. Brain Pathol. 2015, 25, 663–678. [Google Scholar] [CrossRef] [Green Version]
- Wanders, R.J.A. Peroxisomes, lipid metabolism, and peroxisomal disorders. Mol. Genet. Metab. 2004, 83, 16–27. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Waterham, H.R. Peroxisomal disorders: The single peroxisomal enzyme deficiencies. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2006, 1763, 1707–1720. [Google Scholar] [CrossRef] [Green Version]
- Brites, P.; Mooyer, P.A.W.; el Mrabet, L.; Waterham, H.R.; Wanders, R.J.A. Plasmalogens participate in very-long-chain fatty acid-induced pathology. Brain 2009, 132, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Trompier, D.; Vejux, A.; Zarrouk, A.; Gondcaille, C.; Geillon, F.; Nury, T.; Savary, S.; Lizard, G. Brain peroxisomes. Biochimie 2014, 98, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Paik, Y.-K. Genetic deficiency in neuronal peroxisomal fatty acid β-oxidation causes the interruption of dauer development in Caenorhabditis elegans. Sci. Rep. 2017, 7, 9358. [Google Scholar] [CrossRef] [PubMed]
- Kulalert, W.; Kim, D.H. The Unfolded Protein Response in a Pair of Sensory Neurons Promotes Entry of C. elegans into Dauer Diapause. Curr. Biol. 2013, 23, 2540–2545. [Google Scholar] [CrossRef] [Green Version]
- Malone, E.A.; Thomas, J.H. A screen for nonconditional dauer-constitutive mutations in Caenorhabditis elegans. Genetics 1994, 136, 879–886. [Google Scholar] [PubMed]
- Li, W.; Kennedy, S.G.; Ruvkun, G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003, 17, 844–858. [Google Scholar] [CrossRef]
- Kulalert, W.; Sadeeshkumar, H.; Zhang, Y.K.; Schroeder, F.C.; Kim, D.H. Molecular Determinants of the Regulation of Development and Metabolism by Neuronal eIF2α Phosphorylation in Caenorhabditis elegans. Genetics 2017, 206, 251–263. [Google Scholar] [CrossRef]
- Ludewig, A. Ascaroside signaling in C. elegans. WormBook 2013, 1–22. [Google Scholar] [CrossRef] [Green Version]
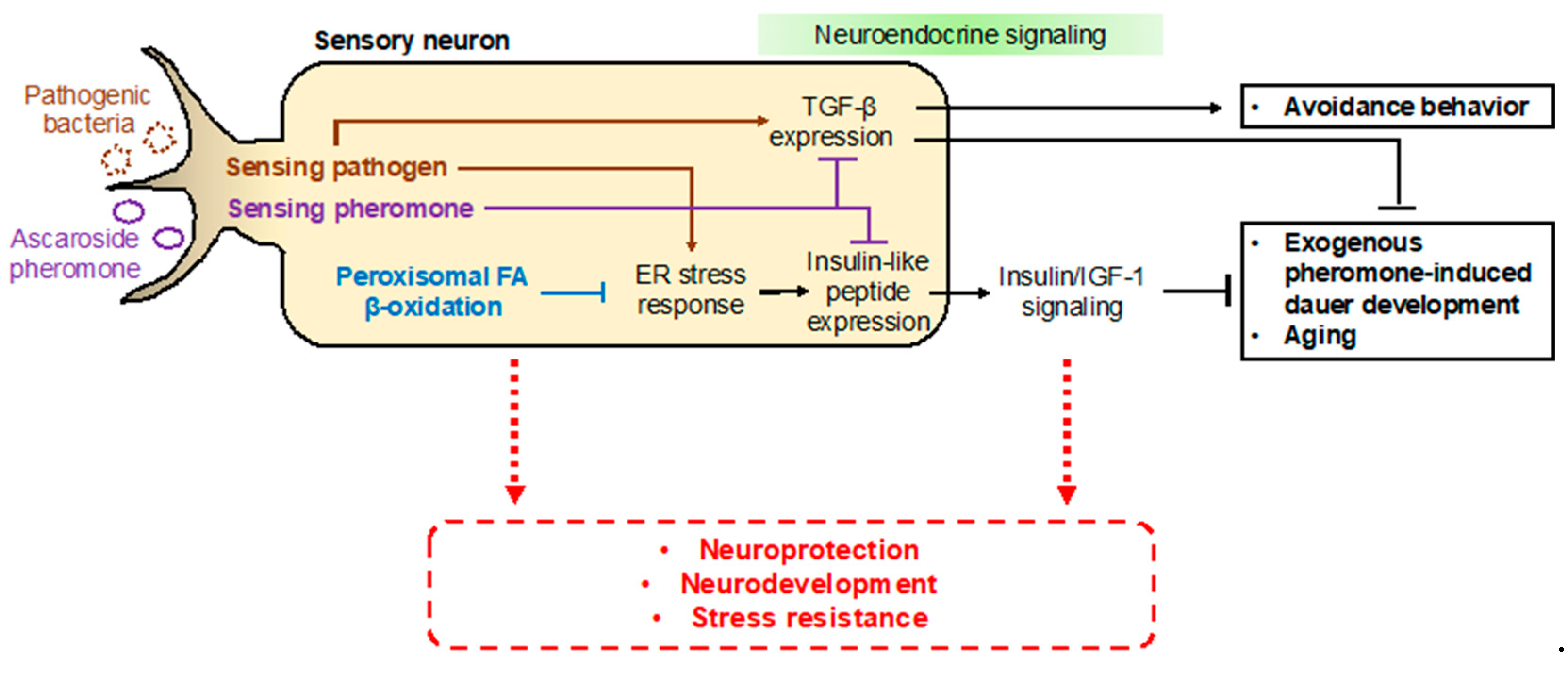
- Neal, S.J.; Takeishi, A.; O’Donnell, M.P.; Park, J.; Hong, M.; Butcher, R.A.; Kim, K.; Sengupta, P. Feeding state-dependent regulation of developmental plasticity via CaMKI and neuroendocrine signaling. eLife 2015, 4, e10110. [Google Scholar] [CrossRef]
- O’Donnell, M.P.; Chao, P.-H.; Kammenga, J.E.; Sengupta, P. Rictor/TORC2 mediates gut-to-brain signaling in the regulation of phenotypic plasticity in C. elegans. PLOS Genet. 2018, 14, e1007213. [Google Scholar] [CrossRef]
- Ilbay, O.; Ambros, V. Pheromones and Nutritional Signals Regulate the Developmental Reliance on let-7 Family MicroRNAs in C. elegans. Curr. Biol. 2019, 29, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Hirotsu, T.; Matsuki, M.; Butcher, R.A.; Tomioka, M.; Ishihara, T.; Clardy, J.; Kunitomo, H.; Iino, Y. Olfactory Plasticity Is Regulated by Pheromonal Signaling in Caenorhabditis elegans. Science 2010, 329, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Fielenbach, N.; Antebi, A.C. elegans dauer formation and the molecular basis of plasticity. Genes Dev. 2008, 22, 2149–2165. [Google Scholar] [CrossRef] [PubMed]
- Hahm, J.-H.; Kim, S.; Paik, Y.-K. Endogenous cGMP regulates adult longevity via the insulin signaling pathway in Caenorhabditis elegans. Aging Cell 2009, 8, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Artan, M.; Jeong, D.-E.; Lee, D.; Kim, Y.-I.; Son, H.G.; Husain, Z.; Kim, J.; Altintas, O.; Kim, K.; Alcedo, J.; et al. Food-derived sensory cues modulate longevity via distinct neuroendocrine insulin-like peptides. Genes Dev. 2016, 30, 1047–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fletcher, M.; Kim, D.H. Age-Dependent Neuroendocrine Signaling from Sensory Neurons Modulates the Effect of Dietary Restriction on Longevity of Caenorhabditis elegans. PLOS Genet. 2017, 13, e1006544. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, A.H.; Gimond, C.; Judkins, J.C.; Thornton, S.; Pulido, D.C.; Micikas, R.J.; Döring, F.; Antebi, A.; Braendle, C.; Schroeder, F.C. Larval crowding accelerates C. elegans development and reduces lifespan. PLOS Genet. 2017, 13, e1006717. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, H.; Ewbank, J.J. The genetics of pathogen avoidance in Caenorhabditis elegans. Mol. Microbiol. 2007, 66, 563–570. [Google Scholar] [CrossRef]
- Meisel, J.D.; Kim, D.H. Behavioral avoidance of pathogenic bacteria by Caenorhabditis elegans. Trends Immunol. 2014, 35, 465–470. [Google Scholar] [CrossRef]
- Kim, D.H. Bacteria and the Aging and Longevity of Caenorhabditis elegans. Annu. Rev. Genet. 2013, 47, 233–246. [Google Scholar] [CrossRef]
- Reddy, K.C.; Andersen, E.C.; Kruglyak, L.; Kim, D.H. A Polymorphism in npr-1 Is a Behavioral Determinant of Pathogen Susceptibility in C. elegans. Science 2009, 323, 382–384. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.C.; Hunter, R.C.; Bhatla, N.; Newman, D.K.; Kim, D.H. Caenorhabditis elegans NPR-1–mediated behaviors are suppressed in the presence of mucoid bacteria. Proc. Natl. Acad. Sci. USA 2011, 108, 12887–12892. [Google Scholar] [CrossRef] [PubMed]
- Styer, K.L.; Singh, V.; Macosko, E.; Steele, S.E.; Bargmann, C.I.; Aballay, A. Innate Immunity in Caenorhabditis elegans Is Regulated by Neurons Expressing NPR-1/GPCR. Science 2008, 322, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Paek, J.; Kim, D.H. Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour. Nature 2011, 480, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Kajino-Sakamoto, R.; Doss, A.; Aballay, A. Distinct Roles of Sensory Neurons in Mediating Pathogen Avoidance and Neuropeptide-Dependent Immune Regulation. Cell Rep. 2017, 21, 1442–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisel, J.D.; Panda, O.; Mahanti, P.; Schroeder, F.C.; Kim, D.H. Chemosensation of Bacterial Secondary Metabolites Modulates Neuroendocrine Signaling and Behavior of C. elegans. Cell 2014, 159, 267–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.S.; Kaletsky, R.; Murphy, C.T. Piwi/PRG-1 Argonaute and TGF-β Mediate Transgenerational Learned Pathogenic Avoidance. Cell 2019, 177, 1827–1841. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Mylonakis, E. An Intestine-Derived Neuropeptide Controls Avoidance Behavior in Caenorhabditis elegans. Cell Rep. 2017, 20, 2501–2512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, C.E.; Kooistra, T.; Kim, D.H. An essential role for XBP-1 in host protection against immune activation in C. elegans. Nature 2010, 463, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.E.; Kinkel, S.; Kim, D.H. Physiological IRE-1-XBP-1 and PEK-1 Signaling in Caenorhabditis elegans Larval Development and Immunity. PLOS Genet. 2011, 7, e1002391. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, Y.; Aballay, A. Organismal regulation of XBP-1-mediated unfolded protein response during development and immune activation. EMBO Rep. 2012, 13, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Singh, V.; Kajino-Sakamoto, R.; Aballay, A. Neuronal GPCR Controls Innate Immunity by Regulating Noncanonical Unfolded Protein Response Genes. Science 2011, 332, 729–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.H.; Chung, H.Y.; Kim, M.; Lee, J.H.; Jung, M.; Ha, H. Daumone fed late in life improves survival and reduces hepatic inflammation and fibrosis in mice. Aging Cell 2014, 13, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ha, H. Short-term Treatment of Daumone Improves Hepatic Inflammation in Aged Mice. Korean J. Physiol. Pharmacol. 2015, 19, 269. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.T.; Lee, S.K.; Park, K.-K.; Park, J.; Son, S.H.; Jung, M.; Chung, W.-Y. Artemisinin-Daumone Hybrid Inhibits Cancer Cell-Mediated Osteolysis by Targeting Cancer Cells and Osteoclasts. Cell. Physiol. Biochem. 2018, 49, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.C. elegans S. Genome Sequence of the Nematode C. elegans: A Platform for Investigating Biology. Science 1998, 282, 2012–2018. [Google Scholar]
- Nagarathnam, B.; Kalaimathy, S.; Balakrishnan, V.; Sowdhamini, R. Cross-Genome Clustering of Human and C. elegans G-Protein Coupled Receptors. Evol. Bioinforma. 2012, 8, EBO–S9405. [Google Scholar] [CrossRef] [PubMed]
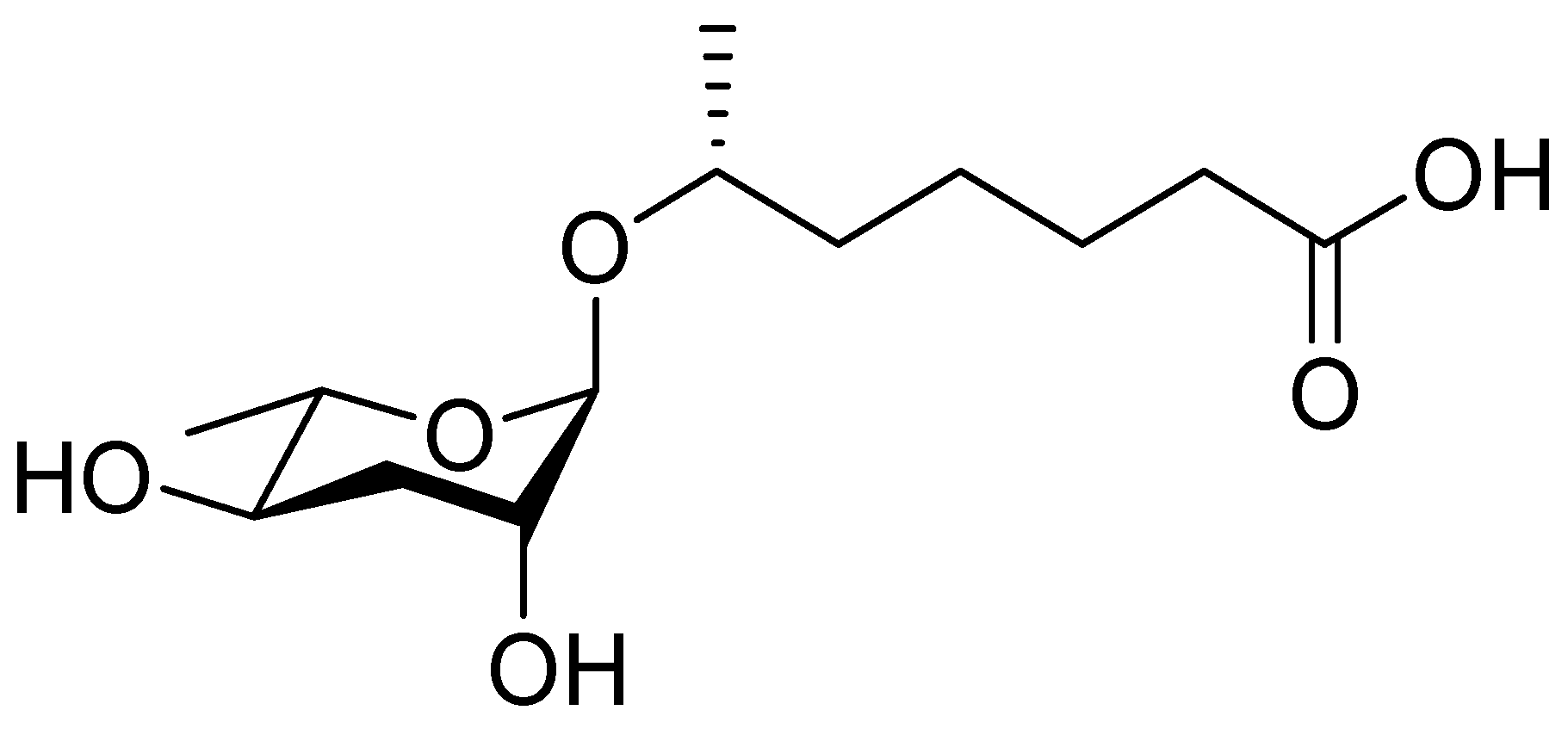

| Name | Chemical Structure | Discovered Receptors | Functions | References |
|---|---|---|---|---|
| ascr#1 |  | SRBC-64 SRBC-66 | Dauer inducing activity Repulsion activity | [13,69,78] |
| ascr#2 |  | DAF-37 DAF-38 SRBC-64 SRBC-66 | Dauer inducing activity Repulsion activity Male attraction activity Foraging activity | [22,26,61,62,69,72,78,82] |
| ascr#3 |  | SRBC-64 SRBC-66 | Dauer inducing activity Repulsion activity Male attraction activity Foraging activity | [22,26,61,62,69,76,77,78,79,80,82] |
| ascr#4 |  | Unknown | Dauer inducing activity Male attraction activity | [26] |
| ascr#5 |  | SRG-36 SRG-37 | Dauer inducing activity Repulsion activity | [23,62,71,82] |
| ascr#6.1 |  | Unknown | Dauer inducing activity | [25] |
| ascr#8 |  | Unknown | Dauer inducing activity Male attraction activity Foraging activity | [25,61,76] |
| icas#3 |  | Unknown | Male attraction activity Aggregation activity | [27] |
| icas#9 |  | SRX-43SRX-44 | Dauer inducing activity Male attraction activity Aggregation activity Foraging activity | [24,27,61,83] |
| hbas#3 |  | Unknown | Hermaphrodite attraction activity | [16] |
| mbas#3 |  | Unknown | Repulsion activity | [16,81] |
| osas#3 |  | Unknown | Repulsion activity | [55] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Joo, H.-J.; Park, S.; Paik, Y.-K. Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions. Int. J. Mol. Sci. 2019, 20, 3898. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20163898
Park JY, Joo H-J, Park S, Paik Y-K. Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions. International Journal of Molecular Sciences. 2019; 20(16):3898. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20163898
Chicago/Turabian StylePark, Jun Young, Hyoe-Jin Joo, Saeram Park, and Young-Ki Paik. 2019. "Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions" International Journal of Molecular Sciences 20, no. 16: 3898. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20163898





