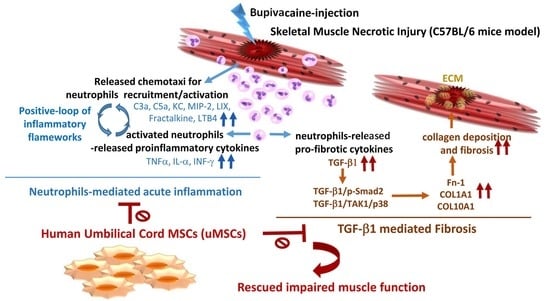
Human Umbilical Cord Mesenchymal Stem Cells Extricate Bupivacaine-Impaired Skeletal Muscle Function via Mitigating Neutrophil-Mediated Acute Inflammation and Protecting against Fibrosis
Abstract
:1. Introduction
2. Result
2.1. Characterization of Mesenchymal Stem Cells Derived from Wharton’s Jelly of Human Umbilical Cord (uMSCs)
2.2. Differentiation of uMSCs into Myoblast-Like Cells In Vitro
2.3. BPVC-Induced Functional Impairment of Gait Was Rescued after Transplantation of uMSCs
2.4. Attenuation of BPVC-Induced Neutrophil Infiltration and Chemotaxis of Neutrophil Recruitment/Activation in Quadriceps Muscles after uMSC Transplantation
2.5. Abrogation of BPVC-Induced Quadriceps Muscle Fibrosis after uMSC Transplantation via Alleviating TGF-β-triggered Canonical Smad2-Dependent and Non-Canonical Smad-Independent TAK1/p38 Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Animals
4.3. Isolation, Cultivation, and Characterization of Human uMSCs
4.4. In Vitro Myogenic Differentiation
4.5. Quadriceps Muscle Injury Induced by Bupivacaine Hydrochloride Injection and Human uMSCs Transplantation
4.6. Cytokine Analysis by Quantibody Mouse Cytokine Array
4.7. Muscle Fibrosis
4.8. Reverse Transcription and Quantitative PCR
4.9. CatWalk Automated Gait Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jarvinen, T.A.; Jarvinen, T.L.; Kaariainen, M.; Kalimo, H.; Jarvinen, M. Muscle injuries: Biology and treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Saul, D.; Boker, K.O.; Ernst, J.; Lehman, W.; Schilling, A.F. Current Methods for Skeletal Muscle Tissue Repair and Regeneration. Biomed. Res. Int. 2018, 2018, 11. [Google Scholar]
- Hurme, T.; Kalimo, H.; Lehto, M.; Jarvinen, M. Healing of skeletal muscle injury: An ultrastructural and immunohistochemical study. Med. Sci. Sports Exerc. 1991, 23, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G. Inflammatory cell response to acute muscle injury. Med. Sci. Sports Exerc. 1995, 27, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Manfredi, T.J.; Ding, W.; Fiatarone, M.A.; Evans, W.J.; Cannon, J.G. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am. J. Physiol. 1993, 265, R166–R172. [Google Scholar] [CrossRef] [PubMed]
- Lammermann, T.; Afonso, P.V.; Angermann, B.R.; Wang, J.M.; Kastenmuller, W.; Parent, C.A.; Germain, R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 2013, 498, 371–375. [Google Scholar]
- Butterfield, T.A.; Best, T.M.; Merrick, M.A. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J. Athl. Train. 2006, 41, 457–465. [Google Scholar] [PubMed]
- Zhu, J.; Li, Y.; Shen, W.; Qiao, C.; Ambrosio, F.; Lavasani, M.; Nozaki, M.; Branca, M.F.; Huard, J. Relationships between transforming growth factor-beta1, myostatin, and decorin: Implications for skeletal muscle fibrosis. J. Biol. Chem. 2007, 282, 25852–25863. [Google Scholar]
- Rizzi, C.F.; Mauriz, J.L.; Freitas Correa, D.S.; Moreira, A.J.; Zettler, C.G.; Filippin, L.I.; Marroni, N.P.; Gonzalez-Gallego, J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg. Med. 2006, 38, 704–713. [Google Scholar]
- Darmani, H.; Crossan, J.; McLellan, S.D.; Meek, D.; Adam, C. Expression of nitric oxide synthase and transforming growth factor-beta in crush-injured tendon and synovium. Mediators Inflamm. 2004, 13, 299–305. [Google Scholar] [Green Version]
- Metsios, G.S.; Stavropoulos-Kalinoglou, A.; Douglas, K.M.; Koutedakis, Y.; Nevill, A.M.; Panoulas, V.F.; Kita, M.; Kitas, G.D. Blockade of tumour necrosis factor-alpha in rheumatoid arthritis: Effects on components of rheumatoid cachexia. Rheumatology (Oxford) 2007, 46, 1824–1827. [Google Scholar] [PubMed]
- McFarlin, K.; Gao, X.; Liu, Y.B.; Dulchavsky, D.S.; Kwon, D.; Arbab, A.S.; Bansal, M.; Li, Y.; Chopp, M.; Dulchavsky, S.A.; et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006, 14, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.F.; Engler, A.J.; Woo, Y.J.; Pirolli, T.J.; Bish, L.T.; Jayasankar, V.; Morine, K.J.; Gardner, T.J.; Discher, D.E.; Sweeney, H.L. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2196–H2203. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, S. Stem cell transplantation for autoimmune diseases: What can we learn from experimental models? Autoimmunity 2008, 41, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Y.; Li, J.T.; Wu, Q.Y.; Li, J.; Feng, Z.T.; Liu, S.; Wang, T.H. Transplantation of NGF-gene-modified bone marrow stromal cells into a rat model of Alzheimer’ disease. J. Mol. Neurosci. 2008, 34, 157–163. [Google Scholar] [PubMed]
- Fu, H.C.; Chuang, I.C.; Yang, Y.C.; Chuang, P.C.; Lin, H.; Ou, Y.C.; Chang Chien, C.C.; Huang, H.S.; Kang, H.Y. Low P16(INK4A) Expression Associated with High Expression of Cancer Stem Cell Markers Predicts Poor Prognosis in Cervical Cancer after Radiotherapy. Int. J. Mol. Sci. 2018, 19, 2541. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.S.; Yang, K.D.; Wang, C.J.; Huang, H.C.; Chio, C.C.; Hsu, T.Y.; Ou, C.Y. Shockwave stimulates oxygen radical-mediated osteogenesis of the mesenchymal cells from human umbilical cord blood. J. Bone Miner. Res. 2004, 19, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Bongso, A.; Fong, C.Y.; Gauthaman, K. Taking stem cells to the clinic: Major challenges. J. Cell Biochem. 2008, 105, 1352–1360. [Google Scholar]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar]
- Nunes, V.A.; Cavacana, N.; Canovas, M.; Strauss, B.E.; Zatz, M. Stem cells from umbilical cord blood differentiate into myotubes and express dystrophin in vitro only after exposure to in vivo muscle environment. Biol. Cell 2007, 99, 185–196. [Google Scholar] [Green Version]
- Conconi, M.T.; Burra, P.; Di Liddo, R.; Calore, C.; Turetta, M.; Bellini, S.; Bo, P.; Nussdorfer, G.G.; Parnigotto, P.P. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int. J. Mol. Med. 2006, 18, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Toumi, H.; F’Guyer, S.; Best, T.M. The role of neutrophils in injury and repair following muscle stretch. J. Anat. 2006, 208, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Korthuis, R.J.; Grisham, M.B.; Granger, D.N. Leukocyte depletion attenuates vascular injury in postischemic skeletal muscle. Am. J. Physiol. 1988, 254, H823–H827. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.B. Initial events in exercise-induced muscular injury. Med. Sci Sports Exerc. 1990, 2, 429–435. [Google Scholar]
- Nonaka, I.; Takagi, A.; Ishiura, S.; Nakase, H.; Sugita, H. Pathophysiology of muscle fiber necrosis induced by bupivacaine hydrochloride (Marcaine). Acta Neuropathol. 1983, 60, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.H. Protein synthesis in bupivacaine (marcaine)-treated, regenerating skeletal muscle. Muscle Nerve 1982, 5, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Moodley, Y.; Atienza, D.; Manuelpillai, U.; Samuel, C.S.; Tchongue, J.; Ilancheran, S.; Boyd, R.; Trounson, A. Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am. J. Pathol. 2009, 175, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Liou, C.W.; Chen, S.D.; Hsu, T.Y.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; Lin, T.K.; et al. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 2015, 22, 31–44. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Liou, C.W.; Chen, S.D.; Wang, P.W.; Chuang, J.H.; Tiao, M.M.; Hsu, T.Y.; Lin, H.Y.; Lin, T.K. Mitochondrial Transfer from Wharton’s Jelly Mesenchymal Stem Cell to MERRF Cybrid Reduces Oxidative Stress and Improves Mitochondrial Bioenergetics. Oxid. Med. Cell Longev. 2017, 2017, 22. [Google Scholar] [CrossRef]
- Molkentin, J.D.; Black, B.L.; Martin, J.F.; Olson, E.N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell 1995, 83, 1125–1136. [Google Scholar] [CrossRef] [Green Version]
- Puri, P.L.; Sartorelli, V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J. Cell Physiol. 2000, 185, 155–173. [Google Scholar] [CrossRef]
- Wurmser, A.E.; Nakashima, K.; Summers, R.G.; Toni, N.; D’Amour, K.A.; Lie, D.C.; Gage, F.H. Cell fusion-independent differentiation of neural stem cells to the endothelial lineage. Nature 2004, 430, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Windrem, M.S.; Nunes, M.C.; Rashbaum, W.K.; Schwartz, T.H.; Goodman, R.A.; McKhann, G., 2nd; Roy, N.S.; Goldman, S.A. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med. 2004, 10, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; von Roth, P.; Matziolis, G.; Schumann, M.R.; Hahn, S.; Strube, P.; Stoltenburg-Didinger, G.; Perka, C.; Duda, G.N.; Tohtz, S.V. Time course of skeletal muscle regeneration after severe trauma. Acta Orthop. 2010, 82, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumucio, J.P.; Sugg, K.B.; Mendias, C.L. TGF-beta superfamily signaling in muscle and tendon adaptation to resistance exercise. Exerc. Sport Sci. Rev. 2015, 43, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Molkentin, J.D.; Bugg, D.; Ghearing, N.; Dorn, L.E.; Kim, P.; Sargent, M.A.; Gunaje, J.; Otsu, K.; Davis, J. Fibroblast-Specific Genetic Manipulation of p38 Mitogen-Activated Protein Kinase In Vivo Reveals Its Central Regulatory Role in Fibrosis. Circulation 2017, 136, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.M.; Chen, Y.H.; Sun, H.S.; Tsai, S.J. Fibroblast growth factors: Potential novel targets for regenerative therapy of osteoarthritis. Chin. J. Physiol. 2019, 62, 2–10. [Google Scholar]
- Kimoto, K.; Nakatsuka, K.; Matsuo, N.; Yoshioka, H. p38 MAPK mediates the expression of type I collagen induced by TGF-beta 2 in human retinal pigment epithelial cells ARPE-19. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2431–2437. [Google Scholar] [CrossRef]
- Rohwedel, J.; Maltsev, V.; Bober, E.; Arnold, H.H.; Hescheler, J.; Wobus, A.M. Muscle cell differentiation of embryonic stem cells reflects myogenesis in vivo: Developmentally regulated expression of myogenic determination genes and functional expression of ionic currents. Dev. Biol. 1994, 164, 87–101. [Google Scholar] [CrossRef]
- Arnold, H.H.; Winter, B. Muscle differentiation: More complexity to the network of myogenic regulators. Curr. Opin. Genet. Dev. 1998, 8, 539–544. [Google Scholar] [CrossRef]
- Weiss, A.; Leinwand, L.A. The mammalian myosin heavy chain gene family. Annu. Rev. Cell Dev. Biol. 1996, 12, 417–439. [Google Scholar] [CrossRef] [PubMed]
- Irintchev, A.; Langer, M.; Zweyer, M.; Theisen, R.; Wernig, A. Functional improvement of damaged adult mouse muscle by implantation of primary myoblasts. J. Physiol. 1997, 500, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Dezawa, M.; Ishikawa, H.; Itokazu, Y.; Yoshihara, T.; Hoshino, M.; Takeda, S.; Ide, C.; Nabeshima, Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science 2005, 309(5732), 314–317. [Google Scholar] [CrossRef] [PubMed]
- Corona, B.T.; Rathbone, C.R. Accelerated functional recovery after skeletal muscle ischemia-reperfusion injury using freshly isolated bone marrow cells. J. Surg. Res. 2014, 188, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.K.; Kekarainen, T.; Heinonen, S.E.; Laitinen, A.; Nystedt, J.; Laine, J.; Yla-Herttuala, S. Umbilical cord blood-derived progenitor cells enhance muscle regeneration in mouse hindlimb ischemia model. Mol. Ther. 2007, 15, 2172–2177. [Google Scholar] [CrossRef] [PubMed]
- Frenette, J.; Cai, B.; Tidball, J.G. Complement activation promotes muscle inflammation during modified muscle use. Am. J. Pathol. 2000, 156, 2103–2110. [Google Scholar] [CrossRef]
- Orimo, S.; Hiyamuta, E.; Arahata, K.; Sugita, H. Analysis of inflammatory cells and complement C3 in bupivacaine-induced myonecrosis. Muscle Nerve 1991, 14, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, R.; Shi, D.; Liu, X.; Chen, Y.; Dou, X.; Zhu, X.; Lu, C.; Liang, W.; Liao, L.; et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood 2009, 113, 46–57. [Google Scholar] [CrossRef]
- Jarvinen, L.; Badri, L.; Wettlaufer, S.; Ohtsuka, T.; Standiford, T.J.; Toews, G.B.; Pinsky, D.J.; Peters-Golden, M.; Lama, V.N. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J. Immunol. 2008, 181, 4389–4396. [Google Scholar] [CrossRef]
- Semple, J.W.; Kim, M.; Hou, J.; McVey, M.; Lee, Y.J.; Tabuchi, A.; Kuebler, W.M.; Chai, Z.W.; Lazarus, A.H. Intravenous immunoglobulin prevents murine antibody-mediated acute lung injury at the level of neutrophil reactive oxygen species (ROS) production. PLoS ONE 2012, 7, e31357. [Google Scholar] [CrossRef]
- Brickson, S.; Hollander, J.; Corr, D.T.; Ji, L.L.; Best, T.M. Oxidant production and immune response after stretch injury in skeletal muscle. Med. Sci. Sports Exerc. 2001, 33, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- St Pierre Schneider, B.; Brickson, S.; Corr, D.T.; Best, T. CD11b+ neutrophils predominate over RAM11+ macrophages in stretch-injured muscle. Muscle Nerve 2002, 25, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Dallegri, F.; Ottonello, L. Tissue injury in neutrophilic inflammation. Inflamm. Res. 1997, 46, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Summan, M.; Gao, X.; Chapman, R.; Hulderman, T.; Simeonova, P.P. Mechanisms of skeletalmuscle injury and repair revealed by gene expression studies in mouse models. J. Physiol. 2007, 582, 825–841. [Google Scholar] [CrossRef] [PubMed]
- Ota, S.; Uehara, K.; Nozaki, M.; Kobayashi, T.; Terada, S.; Tobita, K.; Fu, F.H.; Huard, J. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am. J. Sports Med. 2011, 39, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Stauber, F.; Waters, C.; Alway, S.E.; Stauber, W.T. Transforming growth factor-beta following skeletal muscle strain injury in rats. J. Appl. Physiol. 2007, 102, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, P.; Torchiana, E.; Confalonieri, P.; Brugnoni, R.; Barresi, R.; Mora, M.; Cornelio, F.; Morandi, L.; Mantegazza, R. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J. Clin. Invest. 1995, 96, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Taniguti, A.P.; Pertille, A.; Matsumura, C.Y.; Santo Neto, H.; Marques, M.J. Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-beta1 blocker. Muscle Nerve 2010, 43, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Shinozaki, M.; Hara, T.; Furuya, T.; Miyazono, K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells 2002, 7, 1191–1204. [Google Scholar] [CrossRef]
- Verrecchia, F.; Chu, M.L.; Mauviel, A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 2001, 276, 17058–17062. [Google Scholar] [CrossRef]
- Romaris, M.; Bassols, A.; David, G. Effect of transforming growth factor-beta 1 and basic fibroblast growth factor on the expression of cell surface proteoglycans in human lung fibroblasts. Enhanced glycanation and fibronectin-binding of CD44 proteoglycan, and down-regulation of glypican. Biochem. J. 1995, 310, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Margadant, C.; Sonnenberg, A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010, 11, 97–105. [Google Scholar] [PubMed]
- Iwata, J.; Hacia, J.G.; Suzuki, A.; Sanchez-Lara, P.A.; Urata, M.; Chai, Y. Modulation of noncanonical TGF-beta signaling prevents cleft palate in Tgfbr2 mutant mice. J. Clin. Invest. 2012, 122, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Ida, M.; Takimoto, Y.; Aoyama, T.; Akao, M.; Takeda, T.; Kita, T. Activation of TGF-beta1-TAK1-p38 MAPK pathway in spared cardiomyocytes is involved in left ventricular remodeling after myocardial infarction in rats. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H709–H715. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Meng, K.; Yu, K.; Huang, S.; Huang, Y.; Min, X.; Zhong, Y.; Wu, B.; Liu, Y.; Nie, S.; et al. Exogenous interleukin 37 ameliorates atherosclerosis via inducing the Treg response in ApoE-deficient mice. Sci. Rep. 2017, 7, 3310. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Kessler, H.P. Masson trichrome stain helps differentiate myofibroma from smooth muscle lesions in the head and neck region. J. Formos. Med. Assoc. 2008, 107, 767–773. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, S.; Yu, F.; Li, H.; Guo, C.; Fan, X. Ghrelin Attenuates Liver Fibrosis through Regulation of TGF-beta1 Expression and Autophagy. Int. J. Mol. Sci. 2015, 16, 21911–21930. [Google Scholar] [CrossRef]
- Chuang, P.C.; Wu, M.H.; Shoji, Y.; Tsai, S.J. Downregulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J. Pathol. 2009, 219, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Gwon, D.H.; Kang, D.W.; Hwang, T.W.; Shin, N.; Kwon, H.H.; Shin, H.J.; Yin, Y.; Kim, J.J.; Hong, J.; et al. TLR4-mediated autophagic impairment contributes to neuropathic pain in chronic constriction injury mice. Mol. Brain 2018, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Vrinten, D.H.; Gispen, W.H.; Kalkman, C.J.; Adan, R.A. Interaction between the spinal melanocortin and opioid systems in a rat model of neuropathic pain. Anesthesiology 2003, 99, 449–454. [Google Scholar]





| Gene | NCBI Ref. No | Primer | Sequence |
|---|---|---|---|
| Human Myf5 | NM_005593 | Forward | GCTGCCAGTTCTCACCTTCT |
| Reverse | CACGTGCTCGTCCTCATCT | ||
| Human MyoD | NM_002478 | Forward | TCTCTGCTCCTTTGCCACAAC |
| Reverse | GAGTGCTCTTCGGGTTTCAG | ||
| Human Myogenin | NM_002479 | Forward | AGGTGTGTAAGAGGAAGTCGG |
| Reverse | AGGCGCTCGATGTACTGGA | ||
| Human 18S ribosomal RNA (rRNA) | NR_003286 | Forward | GTGTGCCTACCCTACG |
| Reverse | TGACCCGCACTTACTC | ||
| Mouse GAPDH | NM_008084 | Forward | TTGTGATGGGTGTGAACCAC |
| Reverse | GTCATGAGCCCTTCCACAAT | ||
| Mouse Fn1 | NM_001276413.1 | Forward | AGGAAGCCGAGGTTTTAACTG |
| Reverse | AGGACGCTCATAAGTGTCACC | ||
| Mouse COL1A1 | NM_009912 | Forward | ATGGATTCCCGTTCGAGTACG |
| Reverse | TCAGCTGGATAGCGACATCG | ||
| Mouse COL10A1 | NM_009925 | Forward | GCCAAGCAGTCATGCCTGAT |
| Reverse | GACACGGGCATACCTGTTACC | ||
| Mouse TGF-β | NM_011577 | Forward | GAAGGACCTGGGTTGGAAGT |
| Reverse | TGGTTGTAGAGGGCAAGGAC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, W.-H.; Wang, C.-J.; Fu, H.-C.; Sheng, C.-M.; Tsai, C.-C.; Cheng, J.-H.; Chuang, P.-C. Human Umbilical Cord Mesenchymal Stem Cells Extricate Bupivacaine-Impaired Skeletal Muscle Function via Mitigating Neutrophil-Mediated Acute Inflammation and Protecting against Fibrosis. Int. J. Mol. Sci. 2019, 20, 4312. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174312
Su W-H, Wang C-J, Fu H-C, Sheng C-M, Tsai C-C, Cheng J-H, Chuang P-C. Human Umbilical Cord Mesenchymal Stem Cells Extricate Bupivacaine-Impaired Skeletal Muscle Function via Mitigating Neutrophil-Mediated Acute Inflammation and Protecting against Fibrosis. International Journal of Molecular Sciences. 2019; 20(17):4312. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174312
Chicago/Turabian StyleSu, Wen-Hong, Ching-Jen Wang, Hung-Chun Fu, Chien-Ming Sheng, Ching-Chin Tsai, Jai-Hong Cheng, and Pei-Chin Chuang. 2019. "Human Umbilical Cord Mesenchymal Stem Cells Extricate Bupivacaine-Impaired Skeletal Muscle Function via Mitigating Neutrophil-Mediated Acute Inflammation and Protecting against Fibrosis" International Journal of Molecular Sciences 20, no. 17: 4312. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174312