Bx-daf-22 Contributes to Mate Attraction in the Gonochoristic Nematode Bursaphelenchus xylophilus
Abstract
:1. Introduction
2. Results
2.1. Secretions of Females and Males Attract the Opposite Sex in B. xylophilus
2.2. Ce-daf-22 Duplication Events in B. xylophilus
2.3. Characterization of Bx-daf-22 Genes in B. xylophilus
2.4. Expression Pattern of Bx-daf-22 through the Lifespan of B. Xylophilus
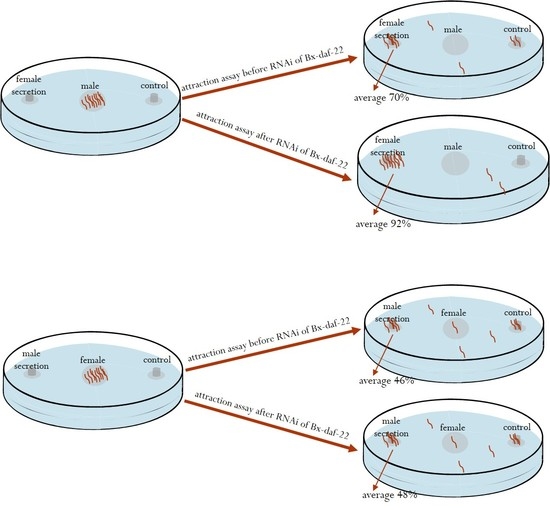
2.5. The Effects of Bx-daf-22 Genes on the Attractiveness of Female and Male Secretions to the Opposite Sex
3. Discussion
4. Materials and Methods
4.1. Nematode Source and Culture Conditions
4.2. Nematode Collection at Different Developmental Stages
4.3. Attraction Assay
4.4. Secretion Attraction Assay
4.5. RNA Extraction and cDNA Synthesis
4.6. Gene Cloning and Phylogenetic Analysis
4.7. Quantitative Real-Time PCR (qRT-PCR) of Nematodes at Different Development Stage
4.8. dsRNA Preparation of Three Bx-daf-22 Genes
4.9. Attraction Assay of Female and Male after Interfering of Bx-daf-22 Genes in B. xylophilus
4.10. Data Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wyatt, T.D. Pheromones and Animal Behaviour: Communication by Smell and Taste; Cambridge University Press: Cambridge, UK, 2003. [Google Scholar]
- Howard, R.W.; Blomquist, G.J. Chemical ecology and biochemistry of insect hydrocarbons. Annu. Rev. Entomol. 1982, 27, 149–172. [Google Scholar]
- Howard, R.W.; Blomquist, G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005, 50, 371–393. [Google Scholar] [PubMed]
- Srinivasan, J.; Kaplan, F.; Ajredini, R.; Zachariah, C.; Alborn, H.T.; Teal, P.E.A.; Malik, R.U.; Edison, A.S.; Sternberg, P.W.; Schroeder, F.C. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008, 454, 1115–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chute, C.D.; Srinivasan, J. Chemical mating cues in C. elegans. Semin. Cell Dev. Biol. 2014, 33, 18–24. [Google Scholar] [PubMed]
- Pungaliya, C.; Srinivasan, J.; Fox, B.W.; Malik, R.U.; Ludewig, A.H.; Sternberg, P.W.; Schroeder, F.C.; Meinwald, J. A shortcut to identifying small molecule signals that regulate behavior and development in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 7708–7713. [Google Scholar] [CrossRef] [PubMed]
- von Reuss, S.H.; Bose, N.; Srinivasan, J.; Yim, J.J.; Judkins, J.C.; Sternberg, P.W.; Schroeder, F.C. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J. Am. Chem. Soc. 2012, 134, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.A. Decoding chemical communication in nematodes. Nat. Prod. Rep. 2017, 34, 472–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butcher, R.A.; Ragains, J.R.; Li, W.; Ruvkun, G.; Clardy, J.; Mak, H.Y. Biosynthesis of the caenorhabditis elegansdauer pheromone. Proc. Natl. Acad. Sci. USA 2009, 106, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Kiontke, K.; Fitch, D. The phylogenetic relationships of Caenorhabditis and other rhabditids. WormBook 2005, 11, 1–11. [Google Scholar]
- Andersson, M.; Simmons, L.W. Sexual selection and mate choice. Trends Ecol. Evol. 2006, 21, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.G.; Ratterman, N.L. Mate choice and sexual selection: what have we learned since Darwin? Proc. Natl. Acad. Sci. 2009, 106, 10001–10008. [Google Scholar] [CrossRef] [PubMed]
- Choe, A.; Chuman, T.; von Reuss, S.H.; Dossey, A.T.; Yim, J.J.; Ajredini, R.; Kolawa, A.A.; Kaplan, F.; Alborn, H.T.; Teal, P.E.A.; et al. Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proc. Natl. Acad. Sci. 2012, 109, 20949–20954. [Google Scholar] [CrossRef] [PubMed]
- Shinya, R.; Morisaka, H.; Takeuchi, Y.; Futai, K.; Ueda, M. Making headway in understanding pine wilt disease: What do we perceive in the postgenomic era? J. Biosci. Bioeng. 2013, 116, 1–8. [Google Scholar] [PubMed]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Yuko, T. Pine Wilt Disease; Bo Guang, Z., Kazuyoshi, F., Jack, R.S., Yuko, T., Eds.; Springer Nature: Basel, Switzerland, 2008. [Google Scholar]
- Zhao, L.; Sun, J. Pinewood Nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle. In Biological Invasions and Its Management in China; Fanghao, W., Mingxing, J., Aibin, Z., Eds.; Springer Nature: Basel, Switzerland, 2017; Volume 2, pp. 3–21. [Google Scholar]
- Jung, J.; Han, H.; Ryu, S.H.; Kim, W. Microsatellite variation in the pinewood nematode, Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle in South Korea. Genes Genomics 2010, 32, 151–158. [Google Scholar]
- Kiyohara, T. Sexual Attraction in Bursaphelenchus xylophilus. Jpn. J. Nematol. 1982, 11, 7–12. [Google Scholar]
- Shinya, R.; Chen, A.; Sternberg, P.W. Sex attraction and mating in Bursaphelenchus okinawaensis and B. xylophilus. J. Nematol. 2015, 47, 176. [Google Scholar] [PubMed]
- Sulston, J.E.; Schierenberg, E.; White, J.G.; Thomson, J. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 1983, 100, 64–119. [Google Scholar] [PubMed]
- Ward, S.; Thomson, N.; White, J.G.; Brenner, S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J. Comp. Neurol. 1975, 160, 313–337. [Google Scholar] [CrossRef]
- Liu, K.S.; Sternberg, P.W. Sensory regulation of male mating behavior in Caenorhabditis elegans. Neuron 1995, 14, 79–89. [Google Scholar] [CrossRef]
- Duggal, C. Sex attraction in the free-living nematode Panagrellus revidivus. Nematologica 1978, 24, 213–221. [Google Scholar] [CrossRef]
- Duggal, C.L. Studies on the Copulatory Behaviour of the Free-Living Nematode Panagrellus Redivivus. Ph.D. Thesis, University of London, London, UK, September 1977. [Google Scholar]
- Simon, J.M.; Sternberg, P.W. Evidence of a mate-finding cue in the hermaphrodite nematode Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2002, 99, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Runnels, A.M.; Murphy, C.T. Mating and male pheromone kill Caenorhabditis males through distinct mechanisms. Elife 2017, 6, e23493. [Google Scholar] [CrossRef] [PubMed]
- Chasnov, J.R.; So, W.K.; Chan, C.M.; Chow, K.L. The species, sex, and stage specificity of a caenorhabditis sex pheromone. Proc. Natl. Acad. Sci. USA 2007, 104, 6730. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.J.; Hu, J.F.; Liu, Z.Y.; Xu, L.; Lu, Q.; Li, Y.X.; Zhang, X.Y. Behavioural features of Bursaphelenchus xylophilus in the mating process. Nematology 2014, 16, 895–902. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Z.Y.; Kai, Z.; Quan, L.; Liang, J.; Zhang, X.Y. Characterization of the Pinus massoniana transcriptional response to Bursaphelenchus xylophilus infection using suppression subtractive hybridization. Int. J. Mol. Sci. 2013, 14, 11356–11375. [Google Scholar]
- Urwin, P.E.; Lilley, C.J.; Atkinson, H.J. Ingestion of double-stranded RNA by preparasitic juvenile cyst nematodes leads to RNA interference. Mol. Plant-Microbe Int. 2002, 15, 747–752. [Google Scholar] [CrossRef]







| Primer Name | Sequences (5′–3′) |
|---|---|
| Bx-daf-22.1F | GTAATCGGAGTGGGTATGAC |
| Bx-daf-22.1R | GAAAGAGCAGCCCAAAGG |
| Bx-daf-22.2F | ATGTCCAAGCCAAAGGTC |
| Bx-daf-22.2R | GCAACAAGTCGTTTGTAG |
| Bx-daf-22.3F | ATGTCCAAGCCAAAAGTC |
| Bx-daf-22.3R | AGCTCAAAGCAAAGTTGTAA |
| DS-daf-22.1-T7F | TAATACGACTCACTATAGGGAATCCTGGTCAGCGAGAAC |
| DS-daf-22.1R | CATAGGTATTGTCTGCTCTGT |
| DS-daf-22.1F | ATCCTGGTCAGCGAGAAC |
| DS-daf-22.1-T7R | TAATACGACTCACTATAGGGACATAGGTATTGTCTGCTCTGT |
| DS-daf-22.2-T7F | TAATACGACTCACTATAGGGAGTGGTTGTGGTCAGTGAG |
| DS-daf-22.2R | ATCAGTTCTCCCGCCTTT |
| DS-daf-22.2F | GTGGTTGTGGTCAGTGAG |
| DS-daf-22.2-T7R | TAATACGACTCACTATAGGGAATCAGTTCTCCCGCCTTT |
| DS-daf-22.3-T7F | TAATACGACTCACTATAGGGATCTATCAACGAGCGGATG |
| DS-daf-22.3R | CAGGATGAAGTCGGAGTC |
| DS-daf-22.3F | TCTATCAACGAGCGGATG |
| DS-daf-22.3-T7R | TAATACGACTCACTATAGGGACAGGATGAAGTCGGAGTC |
| DS-GFP-T7F | TAATACGACTCACTATAGGGATGGTCCCAATTCTCGTGGAAC |
| DS-GFP-R | CTTGAAGTTGACCTTGATGCC |
| DS-GFP-F | TGGTCCCAATTCTCGTGGAAC |
| DS-GFP-T7R | TAATACGACTCACTATAGGGACTTGAAGTTGACCTTGATGCC |
| Actin-QF | TCCGTACCCTGAAGTTGGCTAACC |
| Actin-QR | AAGTGGAGACGAGGGAATGGAACC |
| Bx-daf-22.1QF | CCAATGCGTTGAACTCTG |
| Bx-daf-22.1QR | CCAATACCAATGTTGTGTTGAAGAG |
| Bx-daf-22.2QF | ACATCCTATTGGTGCTACTG |
| Bx-daf-22.2QR | CCAGGTTGTGCTGAAGTC |
| Bx-daf-22.3QF | ATGACTGCTTCGCTTCCA |
| Bx-daf-22.3QR | CGATTCCGAGGTTGTGTT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Li, Y.; Zhang, W.; Wei, P.; Wang, X.; Feng, Y.; Zhang, X. Bx-daf-22 Contributes to Mate Attraction in the Gonochoristic Nematode Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2019, 20, 4316. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174316
Gao M, Li Y, Zhang W, Wei P, Wang X, Feng Y, Zhang X. Bx-daf-22 Contributes to Mate Attraction in the Gonochoristic Nematode Bursaphelenchus xylophilus. International Journal of Molecular Sciences. 2019; 20(17):4316. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174316
Chicago/Turabian StyleGao, Mengge, Yongxia Li, Wei Zhang, Pengfei Wei, Xuan Wang, Yuqian Feng, and Xingyao Zhang. 2019. "Bx-daf-22 Contributes to Mate Attraction in the Gonochoristic Nematode Bursaphelenchus xylophilus" International Journal of Molecular Sciences 20, no. 17: 4316. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20174316