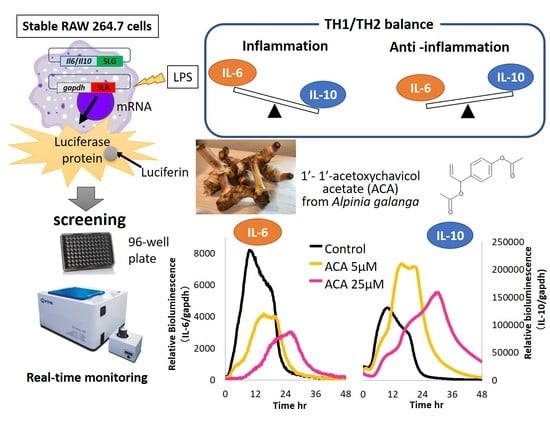
Novel and Stable Dual-Color IL-6 and IL-10 Reporters Derived from RAW 264.7 for Anti-Inflammation Screening of Natural Products
Abstract
:1. Introduction
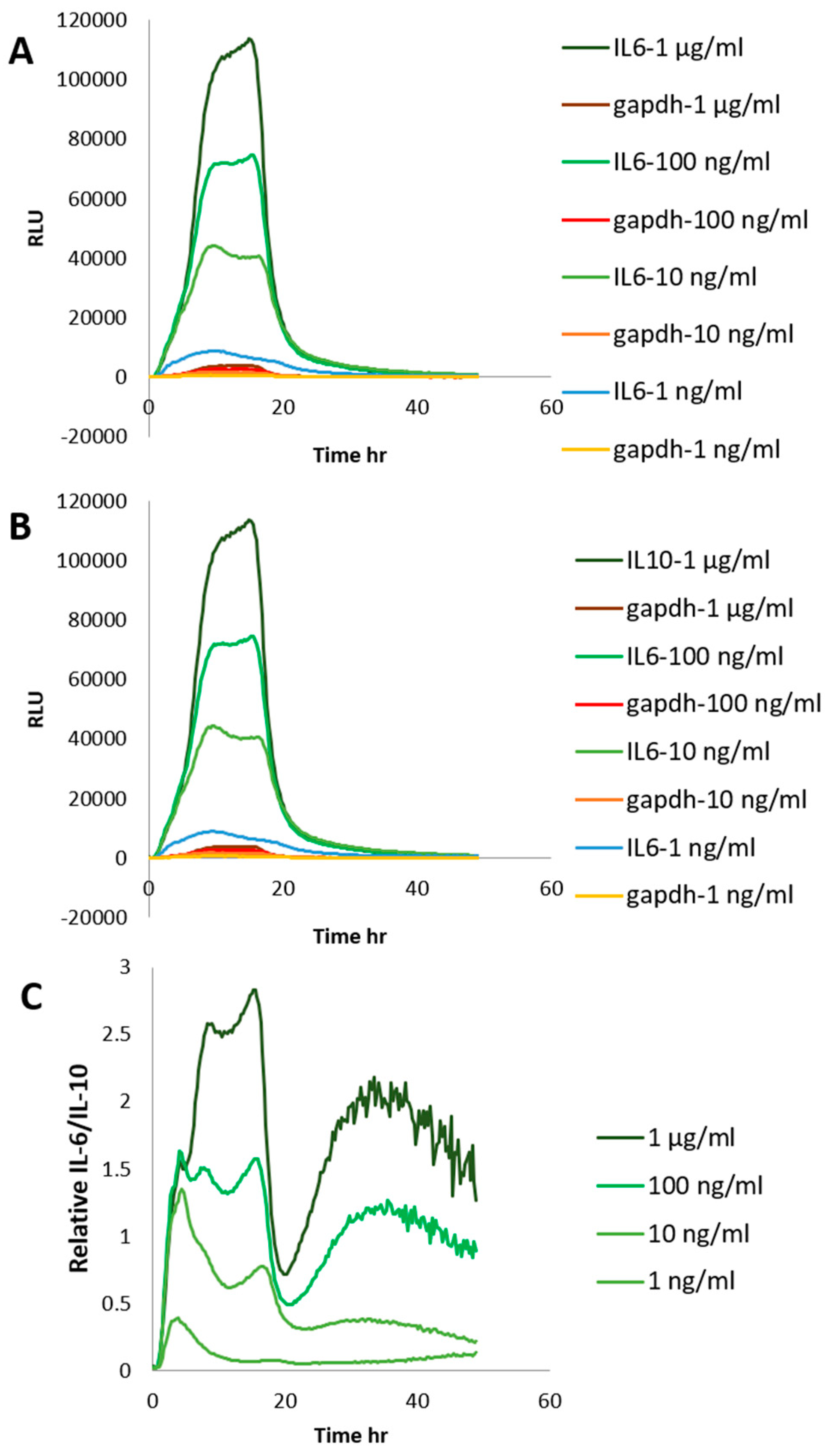
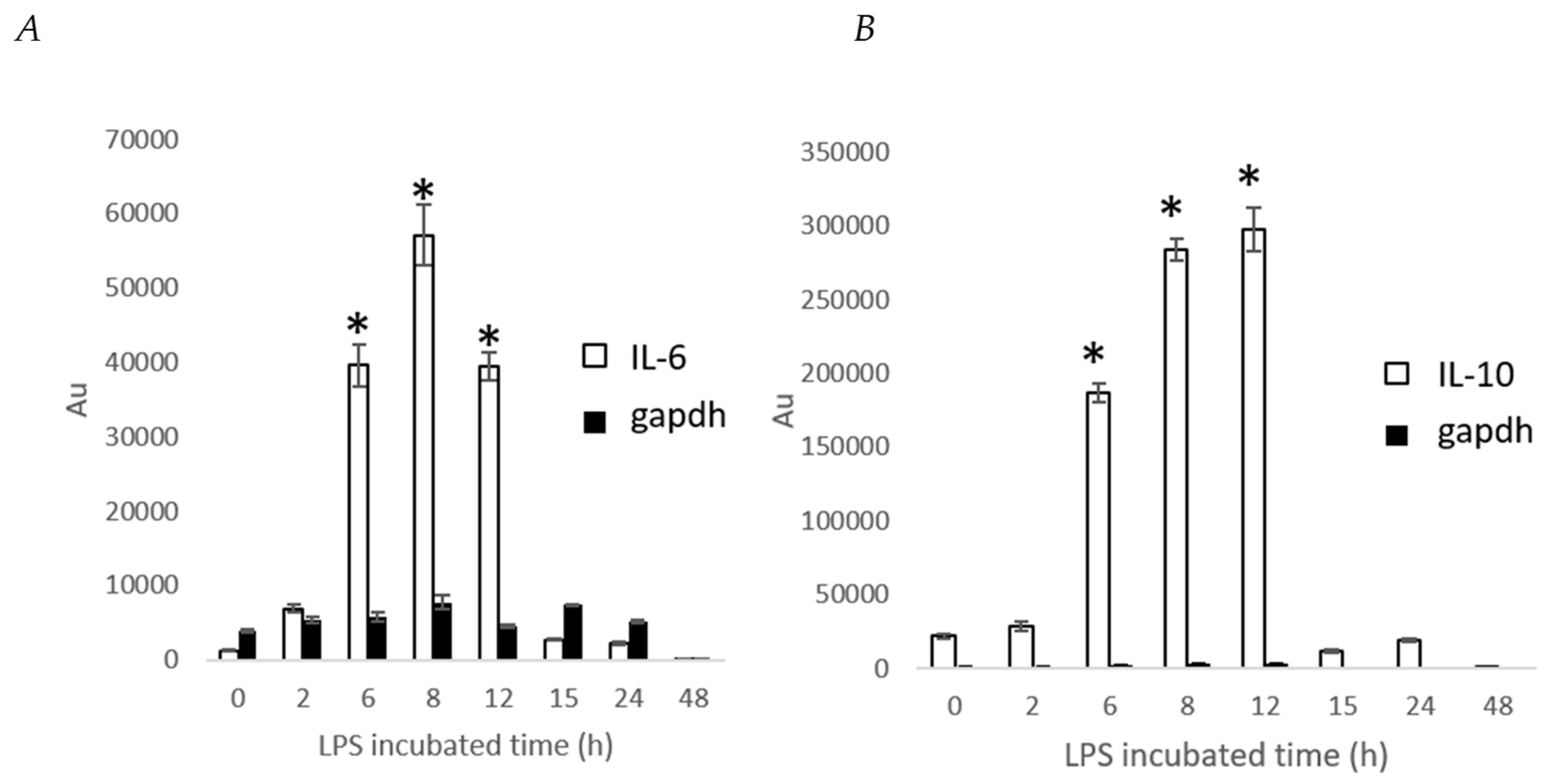
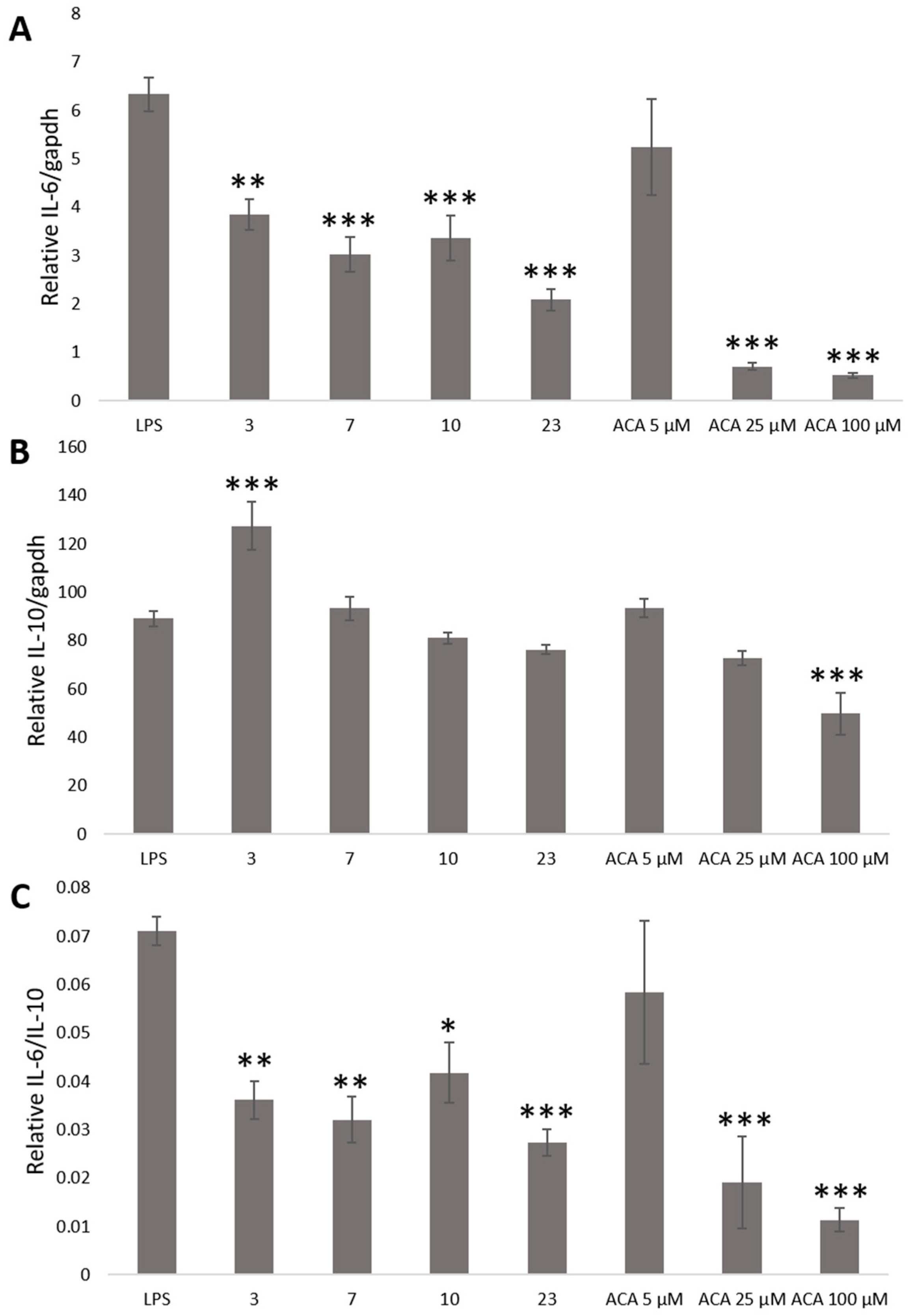
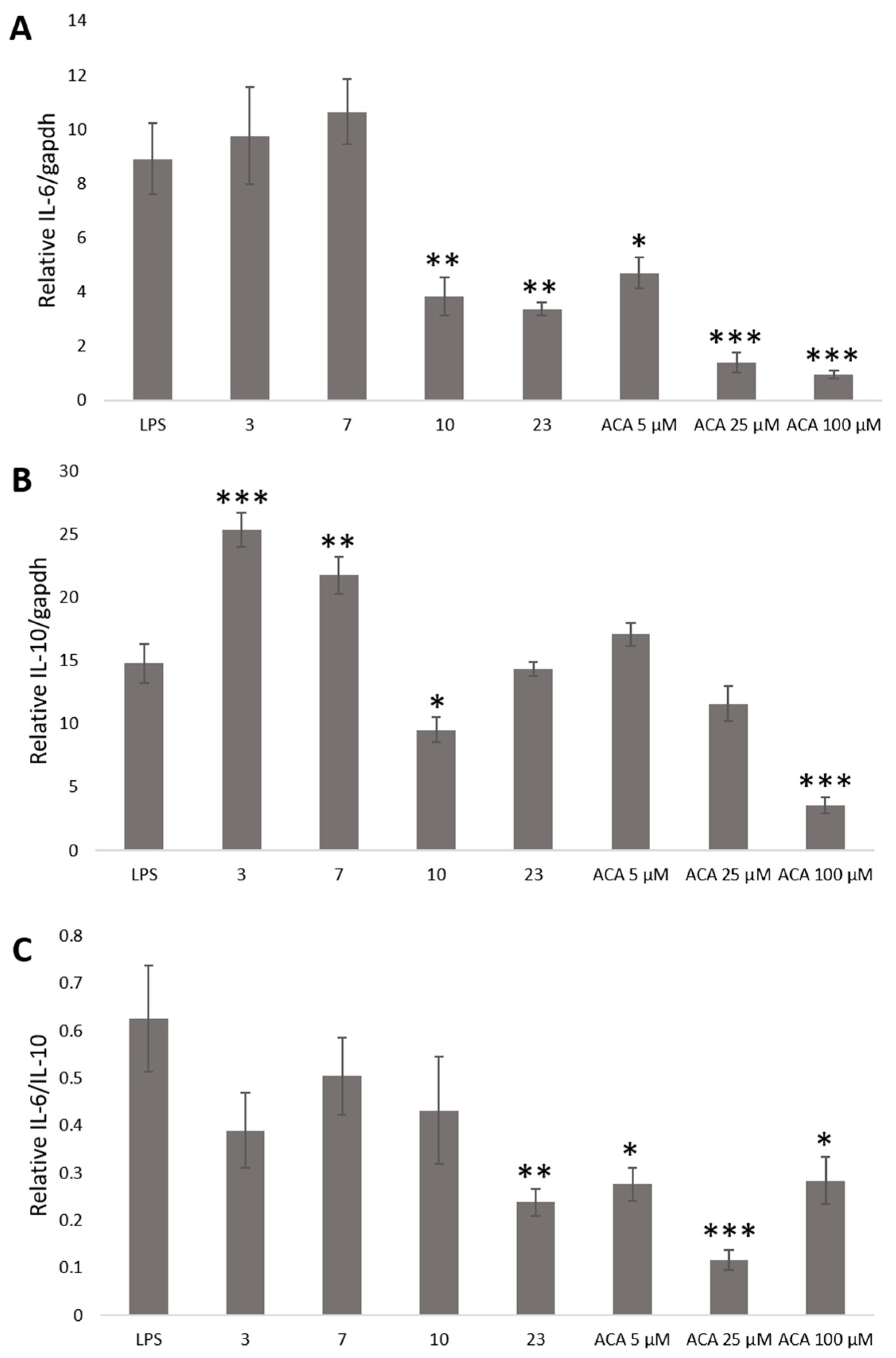
2. Results and Discussion
3. Materials and Methods
3.1. Cell Culture
3.2. Extraction, Isolation, and Purification of 1′S-1′-Acetoxychavicol Acetate
3.3. Analysis of Nuclear Magnetic Resonance Spectroscopy (NMR)
3.4. Nitric Oxide (NO) Production
3.5. Cell Proliferation Assay
3.6. Generation of Stable RAW 264.7 Cell Lines
3.7. Determination IL-6 and IL-10 Level by Stable Cells Using a Bioluminescence Measurement System for Living Cells by Real-Time Monitoring
3.8. Determination IL-6 and IL-10 Level by Stable Cells Using a Microplate Luminometer
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Esch, T.; Stefano, G.B.; Fricchione, G.L.; Benson, H. Stress-related diseases—A potential role for nitric oxide. Med. Sci. Monit. 2002, 8, 103–118. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Soufli, I.; Toumi, R.; Rafa, H.; Touil-Boukoffa, C. Overview of cytokines and nitric oxide involvement in immuno-pathogenesis of inflammatory bowel diseases. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Saiki, P.; Kawano, Y.; Van Griensven, L.; Miyazaki, K. The anti-inflammatory effect of Agaricus brasiliensis is partly due to its linoleic acid content. Food Funct. 2017, 8, 4150–4158. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [PubMed]
- Atwell, D.M.; Grichnik, K.P.; Newman, M.F.; Reves, J.G.; McBride, W.T. Balance of proinflammatory and antiinflammatory cytokines at thoracic cancer operation. Ann. Thorac. Surg. 1998, 66, 1145–1150. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Kunz, M.; Cereser, K.M.; Goi, P.D.; Fries, G.R.; Teixeira, A.L.; Fernandes, B.S.; Belmonte-de-Abreu, P.S.; Kauer-Sant’Anna, M.; Kapczinski, F.; Gama, C.S. Serum levels of IL-6, IL-10 and TNF-alpha in patients with bipolar disorder and schizophrenia: Differences in pro- and anti-inflammatory balance. Braz. J. Psychiatry 2011, 33, 268–274. [Google Scholar]
- Wallinder, J.; Bergqvist, D.; Henriksson, A.E. Proinflammatory and anti-inflammatory cytokine balance in patients with abdominal aortic aneurysm and the impact of aneurysm size. Vasc. Endovasc. Surg. 2009, 43, 258–261. [Google Scholar] [CrossRef]
- Niu, Q.; Cai, B.; Huang, Z.C.; Shi, Y.Y.; Wang, L.L. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2731–2736. [Google Scholar] [CrossRef]
- Uhm, W.S.; Na, K.; Song, G.W.; Jung, S.S.; Lee, T.; Park, M.H.; Yoo, D.H. Cytokine balance in kidney tissue from lupus nephritis patients. Rheumatology 2003, 42, 935–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suabjakyong, P.; Nishimura, K.; Toida, T.; Van Griensven, L.J. Structural characterization and immunomodulatory effects of polysaccharides from Phellinus linteus and Phellinus igniarius on the IL-6/IL-10 cytokine balance of the mouse macrophage cell lines (RAW 264.7). Food Funct. 2015, 6, 2834–2844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, Y.; Kimura, T.; Sugata, K.; Enomoto, T.; Asakawa, A.; Kubota, H.; Ikeda, M.; Ohmiya, Y. Multicolor luciferase assay system: One-step monitoring of multiple gene expressions with a single substrate. Biotechniques 2005, 38, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, Y. Simultaneous multicolor luciferase reporter assays for monitoring of multiple genes expressions. Comb. Chem. High. Throughput Screen 2015, 18, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Toledo, J.C., Jr.; Augusto, O. Connecting the chemical and biological properties of nitric oxide. Chem. Res. Toxicol. 2012, 25, 975–989. [Google Scholar] [CrossRef]
- Saiki, P.; Nakajima, Y.; Van Griensven, L.; Miyazaki, K. Real-time monitoring of IL-6 and IL-10 reporter expression for anti-inflammation activity in live RAW 264.7cells. Biochem. Biophys Res. Commun. 2018, 505, 885–890. [Google Scholar] [CrossRef]
- Takahashi, T.; Kimura, Y.; Saito, R.; Nakajima, Y.; Ohmiya, Y.; Yamasaki, K.; Aiba, S. An in vitro test to screen skin sensitizers using a stable THP-1-derived IL-8 reporter cell line, THP-G8. Toxicol. Sci. 2011, 124, 359–369. [Google Scholar] [CrossRef]
- Chatterjee, S.; Niaz, Z.; Gautam, S.; Adhikari, S.; Variyar, P.S.; Sharma, A. Antioxidant activity of some phenolic constituents from green pepper (Piper nigrum L.) and fresh nutmeg mace (Myristica fragrans). Food Chem. 2007, 101, 515–523. [Google Scholar] [CrossRef]
- Calliste, C.; Kozlowski, D.; Duroux, J.; Champavier, Y.; Chulia, A.; Trouillas, P. A new antioxidant from wild nutmeg. Food Chem. 2010, 118, 489–496. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Akanji, M.A.; Hafizur, R.M.; Choudhary, M.I. Antiglycation, antioxidant and toxicological potential of polyphenol extracts of alligator pepper, ginger and nutmeg from Nigeria. Asian Pac. J. Trop. Biomed. 2012, 2, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.-P.; Wu, H.; Wang, Y.; Weng, X.-C. Isolation of some compounds from nutmeg and their antioxidant activities. Czech J. Food Sci. 2012, 30, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Vallverdú-Queralt, A.; Regueiro, J.; Alvarenga, J.F.R.; Martinez-Huelamo, M.; Leal, L.N.; Lamuela-Raventos, R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015, 35, 189–195. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Takikawa, A.; Abe, K.; Yamamoto, M.; Ishimaru, S.; Yasui, M.; Okubo, Y.; Yokoigawa, K. Antimicrobial activity of nutmeg against Escherichia coli O157. J. Biosci. Bioeng 2002, 94, 315–320. [Google Scholar] [CrossRef]
- Morita, T.; Jinno, K.; Kawagishi, H.; Arimoto, Y.; Suganuma, H.; Inakuma, T.; Sugiyama, K. Hepatoprotective effect of myristicin from nutmeg (Myristica fragrans) on lipopolysaccharide/d-galactosamine-induced liver injury. J. Agric. Food Chem. 2003, 51, 1560–1565. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, X.W.; Krausz, K.W.; Nichols, R.G.; Xu, W.; Patterson, A.D.; Gonzalez, F.J. Modulation of colon cancer by nutmeg. J. Proteome Res. 2015, 14, 1937–1946. [Google Scholar] [CrossRef] [PubMed]
- Kareem, M.A.; Krushna, G.S.; Hussain, S.A.; Devi, K.L. Effect of aqueous extract of nutmeg on hyperglycaemia, hyperlipidaemia and cardiac histology associated with isoproterenol-induced myocardial infarction in rats. Trop. J. Pharm. Res. 2009, 8, 337–344. [Google Scholar] [CrossRef]
- Radwan, M.M.; Tabanca, N.; Wedge, D.E.; Tarawneh, A.H.; Cutler, S.J. Antifungal compounds from turmeric and nutmeg with activity against plant pathogens. Fitoterapia 2014, 99, 341–346. [Google Scholar] [CrossRef]
- Baker, I.; Chohan, M.; Opara, E.I. Impact of cooking and digestion, in vitro, on the antioxidant capacity and anti-inflammatory activity of cinnamon, clove and nutmeg. Plant Foods Hum. Nutr. 2013, 68, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Dewi, K.; Widyarto, B.; Erawijantari, P.P.; Widowati, W. In vitro study of Myristica fragrans seed (Nutmeg) ethanolic extract and quercetin compound as anti-inflammatory agent. Int. J. Res. Med. Sci. 2015, 3, 2303–2310. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, W. Anti-inflammatory effect of myristicin on RAW 264.7 macrophages stimulated with polyinosinic-polycytidylic acid. Molecules 2011, 16, 7132–7142. [Google Scholar] [CrossRef] [PubMed]
- Baradwaj, R.G.; Rao, M.V.; Senthil Kumar, T. Novel purification of 1′S-1′-Acetoxychavicol acetate from Alpinia galanga and its cytotoxic plus antiproliferative activity in colorectal adenocarcinoma cell line SW480. Biomed. Pharmacother. 2017, 91, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Awang, K.; Azmi, M.N.; Aun, L.I.; Aziz, A.N.; Ibrahim, H.; Nagoor, N.H. The apoptotic effect of 1′s-1′-acetoxychavicol acetate from Alpinia conchigera on human cancer cells. Molecules 2010, 15, 8048–8059. [Google Scholar] [CrossRef] [PubMed]
- Phuah, N.H.; In, L.L.; Azmi, M.N.; Ibrahim, H.; Awang, K.; Nagoor, N.H. Alterations of microRNA expression patterns in human cervical carcinoma cells (Ca Ski) toward 1′S-1′-acetoxychavicol acetate and cisplatin. Reprod Sci. 2013, 20, 567–578. [Google Scholar] [CrossRef]
- Nagoor, N.H.; Lionel, I.; Nazif, A.; Eswary, T.; Halijah, I.; Awang, K. The cytotoxic and Apoptotic efects of 1′s-1′-acetoxychavicol acetate from alpinia conchigera griff on various human tumour cell lines. In Proceedings of the International Trade Exhibition and Conference for Biotechnology, Bangkok, Thailand, 25–27 November 2008; Faculty of Science, University of Malaya: Kuala Lumpur, Malaysia, 2008. [Google Scholar]
- Mohd Arshad, N. Anticancer Effects of 1′s-1′-Acetoxychavicol Acetate and Its Potentiation When Conjugated with Human Alpha Fetoprotein against Human Tumours in Mice/Norhafiza Binti Mohd Arshad. Ph.D. Thesis, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia, 2015. [Google Scholar]
- Phuah, N.H. Determination of the Role of miR-210 AND miR-629 in Regulating Response towards 1′S-1′-Acetoxychavicol Acetate in Human Cervical Carcinoma Cells CaSki AND SiHa/Phuah Neoh Hun. Ph.D. Thesis, Faculty of Science, University of Malaya, Kuala Lumpur, Malaysia, 2017. [Google Scholar]
- Ohata, T.; Fukuda, K.; Murakami, A.; Ohigashi, H.; Sugimura, T.; Wakabayashi, K. Inhibition by 1′-acetoxychavicol acetate of lipopolysaccharide-and interferon-gamma-induced nitric oxide production through suppression of inducible nitric oxide synthase gene expression in RAW264 cells. Carcinogenesis 1998, 19, 1007–1012. [Google Scholar] [CrossRef]
- Noguchi, T.; Michihata, T.; Nakamura, W.; Takumi, T.; Shimizu, R.; Yamamoto, M.; Ikeda, M.; Ohmiya, Y.; Nakajima, Y. Dual-color luciferase mouse directly demonstrates coupled expression of two clock genes. Biochemistry 2010, 49, 8053–8061. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef]

| NO Production | Relative IL-6/Gapdh | Relative IL-10/Gapdh | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Extract Conc. (μg/mL) | Extract Conc. (μg/mL) | Extract Conc. (μg/mL) | ||||||||
| No. | Sample Name | 150 | 100 | 50 | 75 | 50 | 25 | 75 | 50 | 25 |
| 1 | cinnamon | 0 | 0 | ● | ● | ● | ||||
| 2 | turmeric | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 | nutmeg | 0 | 0 | 0 | 0 | 0 | ● | ● | ● | |
| 4 | black pepper | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 5 | white pepper | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 6 | red pepper | ● | ● | ● | ||||||
| 7 | oregano | 0 | 0 | ● | ● | |||||
| 8 | basil | ● | ||||||||
| 9 | parsley | 0 | ● | ● | ● | |||||
| 10 | laurel | 0 | 0 | 0 | 0 | 0 | 0 | ● | ● | ● |
| 11 | cardamom | 0 | ● | |||||||
| 12 | cumin | 0 | ● | ● | ||||||
| 13 | cilantro | 0 | ● | ● | ● | |||||
| 14 | fennel | 0 | 0 | 0 | ● | ● | ● | |||
| 15 | anise seed | 0 | 0 | 0 | ● | ● | ● | |||
| 16 | allspice | ● | ● | ● | ||||||
| 17 | caraway | ● | ● | ● | ||||||
| 18 | clove | 0 | ||||||||
| 19 | Japanese pepper | 0 | 0 | 0 | ||||||
| 20 | ginger | 0 | ● | ● | ● | |||||
| 21 | star anise | ● | ||||||||
| 22 | celery seed | ● | ● | ● | ||||||
| 23 | long Pepper | 0 | 0 | 0 | 0 | 0 | ● | ● | ● | |
| 24 | fenogreek | ● | ● | ● | ||||||
| 25 | mace | 0 | 0 | 0 | ● | ● | ● | |||
| 26 | dil Seed | ● | ● | ● | ||||||
| 27 | kaffir lime leaf | 0 | 0 | 0 | ● | ● | ● | |||
| 28 | summer savory | ● | ● | |||||||
| 29 | kaba | 0 | 0 | ● | ● | ● | ||||
| 30 | sage | 0 | 0 | |||||||
| 31 | thyme | ● | ● | ● | ||||||
| 32 | taragon | 0 | ● | ● | ● | |||||
| 33 | marjoram | ● | ● | ● | ||||||
| 34 | rosemary | 0 | 0 | 0 | 0 | |||||
| 35 | Chinese pepper | 0 | 0 | 0 | 0 | |||||
| 36 | liquorice | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 37 | garlic | ● | ● | ● | ||||||
| 38 | green Pepper | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 39 | paprika | ● | ● | ● | ||||||
| 40 | horse radish | ● | ● | ● | ||||||
| 41 | mustard | ● | ● | ● | ||||||
| 42 | curry leaf | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 43 | spearmint | |||||||||
| 44 | dillweed | |||||||||
| 45 | peppermint | 0 | 0 | |||||||
| 46 | lemongrass | 0 | 0 | 0 | 0 | 0 | ||||
| 47 | Saffron | |||||||||
| extract conc. (μg/mL) | 20 | 10 | 5 | |||||||
| 48 | greater galangal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ● |
| extract conc. (μg/mL) | 150 | 100 | 50 | |||||||
| 49 | crude polysaccharide extract from Agaricus b. | ● | ● | ● | ||||||
| 50 | crude polyphenol extract. from Agaricus b | 0 | 0 | 0 | ● | ● | ● | |||
| 51 | crude polysaccharide extract from Ganoderma l. | ● | ● | ● | ||||||
| 52 | crude polysaccharide extract from Phellinus l. | 0 | 0 | 0 | 0 | 0 | ||||
| 0 | decreased | |||||||||
| ● | increased | |||||||||
| ACA | 1.3 | ACA | 1.3 | |
|---|---|---|---|---|
| A. Khalijah, et al. | H. Azuma et al. | |||
| Carbon Number | 13C (ppm) | 13C (ppm) | 1H (ppm) | 1H (ppm) |
| 1 | 150.5, s | 150.6, s | ||
| OCOCH3 | 169.4, s | 169.4, s | ||
| OCOCH3 | 21.2, q | 21.2, q | 2.29 (s, 3H) | 2.25 (s, 3H) |
| 2 | 121.7, d | 121.8, d | 7.08 (d, 8.5, 1H) | 7.07 (d, 8.5, 1H) |
| 3 | 128.5, d | 128.6, d | 7.37 (d, 8.5, 1H) | 7.36 (d, 8.5, 1H) |
| 4 | 136.5, s | 136.6, s | ||
| 5 | 128.5, d | 128.6, d | 7.37 (d, 8.5, 1H) | 7.36 (d, 8.5, 1H) |
| 6 | 121.7, d | 121.8, d | 7.08 (d, 8.5 1H) | 7.07 (d, 8.5, 1H) |
| 1′ | 75.6, d | 75.6, d | 6.26 (d, 5.9, 1H) | 6.26 (d, 5.9, 1H) |
| OCOCH3 | 169.7, s | 169.8, s | ||
| OCOCH3 | 21.3, q | 21.3, q | 2.10 (s, 3H) | 2.10 (s, 3H) |
| 2′ | 136.1, d | 136.2, d | 5.98 (ddd, 17.1, 10.5, 5.9, 1H) | 5.98 (ddd, 17.1, 10.7, 6.1, 1H) |
| 3′ | 117.2, t | 117.2, t | 5.30 (d, 17.1, 1H), 5.25 (d, 10.5, 1H) | 5.29 (d, 17.1, 1H), 5.23 (d, 10.5, 1H) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiki, P.; Kawano, Y.; Nakajima, Y.; Van Griensven, L.J.L.D.; Miyazaki, K. Novel and Stable Dual-Color IL-6 and IL-10 Reporters Derived from RAW 264.7 for Anti-Inflammation Screening of Natural Products. Int. J. Mol. Sci. 2019, 20, 4620. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184620
Saiki P, Kawano Y, Nakajima Y, Van Griensven LJLD, Miyazaki K. Novel and Stable Dual-Color IL-6 and IL-10 Reporters Derived from RAW 264.7 for Anti-Inflammation Screening of Natural Products. International Journal of Molecular Sciences. 2019; 20(18):4620. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184620
Chicago/Turabian StyleSaiki, Papawee, Yasuhiro Kawano, Yoshihiro Nakajima, Leo J. L. D. Van Griensven, and Koyomi Miyazaki. 2019. "Novel and Stable Dual-Color IL-6 and IL-10 Reporters Derived from RAW 264.7 for Anti-Inflammation Screening of Natural Products" International Journal of Molecular Sciences 20, no. 18: 4620. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184620