Instrument-Free and Visual Detection of Salmonella Based on Magnetic Nanoparticles and an Antibody Probe Immunosensor
Abstract
:1. Introduction
2. Results
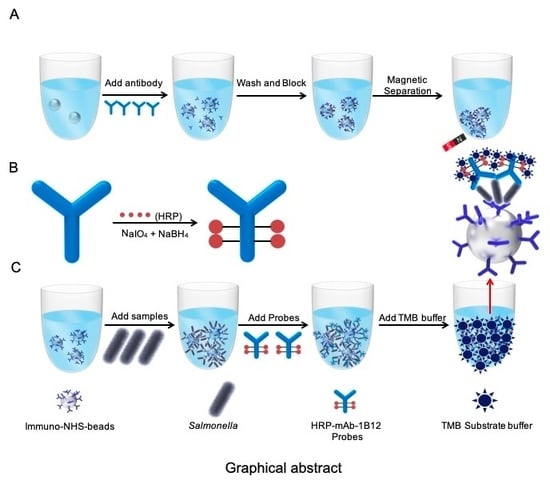
2.1. Antibody-Probe-Based Immuno-N-Hydroxysuccinimide (NHS) bead (AIB) System Design
2.2. Generation of Specific mAbs Against Salmonella
2.3. Synthesis of the HRP mAb Probes
2.4. Characterization of the Paired Antibodies
2.5. INB Preparation and Characterization
2.6. AIB System Optimization
2.7. Assessment of Salmonella Detection Using the AIB System
2.8. Salmonella Detection by the AIB System in Artificially Contaminated Samples
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Production of mAbs Against Salmonella
4.3. Purification of Ascites
4.4. ELISA and Western Blot Assay
4.5. HRP mAb Probe Preparation and Characterization
4.6. INB Preparation and Characterization
4.7. Performance of the AIB System
4.8. System Evaluation using Contaminated Samples
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIB | antibody-probe-based immuno-N-hydroxysuccinimide beads |
| DAS-ELISA | double sandwich ELISA |
| ELISA | enzyme-linked immunosorbant assay |
| FBS | fatal bovine serum |
| FCA | Freund’s complete adjuvant |
| FIA | Freund’s incomplete adjuvant |
| IC-PCR | immunocaptured-PCR |
| INBs | immuno-NHS beads |
| LAMP | loop-mediated isothermal amplification assay |
| mAb | monoclonal antibody |
| NHS beads | N-hydroxysuccinimide (NHS) beads |
| PCR | polymerase chain reaction |
| HAT | hypoxanthine–aminopterin–thymidine |
| HRP | horse radish peroxidase |
| HT | hypoxanthine–thymidine |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TMB | 3, 3′, 5, 5′-tetramethylbenzidine |
References
- Alves, J.; Niguma, N.H.; de Oliveira, T.C.R.M. Detection of Salmonella spp. in Eight Complex Food Matrices Using Polymerase Chain Reaction Assay. Int. J. Mol. Sci. 2015, 35, 453–457. [Google Scholar] [CrossRef]
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases - the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microblol. 2010, 139, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Salzman, N.H.; Ghosh, D.; Huttner, K.M.; Paterson, Y.; Bevins, C.L. Protection against enteric salmonellosis in transgenic mice expressing a human intestinal defensin. Nature 2003, 422, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kingsley, R.A.; Santos, R.L.; Andrews-Polymenis, H.; Raffatellu, M.; Figueiredo, J.; Nunes, J.; Tsolis, R.M.; Adams, L.G.; Baumler, A.J. Molecular pathogenesis of Salmonella enterica serotype typhimurium-induced diarrhea. Infect. Immu. 2003, 71, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Switt, A.I.; Soyer, Y.; Warnick, L.D.; Wiedmann, M. Emergence, distribution, and molecular and phenotypic characteristics of Salmonella enterica serotype 4, 5, and 12:1. Foodborne Pathog. Dis. 2009, 6, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Abdoel, T.H.; Pastoor, R.; Smits, H.L.; Hatta, M. Laboratory evaluation of a simple and rapid latex agglutination assay for the serodiagnosis of typhoid fever. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Paul, M.; Goldberg, E.; Acosta, C.J.; Leibovici, L. Typhoid fever vaccines: Systematic review and meta-analysis of randomised controlled trials. Vaccine 2007, 25, 7848–7857. [Google Scholar] [CrossRef]
- Liu, H.B.; Zang, Y.X.; Du, X.J.; Li, P.; Wang, S. Development of an isothermal amplification-based assay for the rapid visual detection of Salmonella bacteria. J. Dairy Sci. 2017, 100, 7016–7025. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Zhu, S.; Jin, C.; Chen, F. Development of multiplex loop-mediated isothermal amplification-RFLP (mLAMP-RFLP) to detect Salmonella spp. and Shigella spp. in milk. Int. J. Food Microbiol. 2011, 148, 75–79. [Google Scholar] [CrossRef]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Inf. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef]
- Kuang, H.; Cui, G.; Chen, X.; Yin, H.; Yong, Q.; Xu, L.; Peng, C.; Wang, L.; Xu, C. A one-step homogeneous sandwich immunosensor for Salmonella detection based on magnetic nanoparticles (MNPs) and quantum Dots (QDs). Int. J. Mol. Sci. 2013, 14, 8603–8610. [Google Scholar] [CrossRef] [PubMed]
- Khueankhancharoen, J.; Thipayarat, A.; Saranak, J. Optimized microscale detection of amino acid decarboxylase for rapid screening of Salmonella in the selective enrichment step. Food Control. 2016, 69, 352–367. [Google Scholar] [CrossRef]
- Chin, W.H.; Sun, Y.; Hogberg, J.; Quyen, T.L.; Engelsmann, P.; Wolff, A.; Bang, D.D. Direct PCR-A rapid method for multiplexed detection of different serotypes of Salmonella in enriched pork meat samples. Mol. Cell. Probes. 2017, 32, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Domesle, K.J.; Yang, Q.; Hammack, T.S.; Ge, B. Validation of a Salmonella loop-mediated isothermal amplification assay in animal food. Int. J. Food Microbiol. 2018, 264, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, C.T.; Jeffery, K.J.M.; Bowler, I. Stool PCR may not be a substitute for enrichment culture for the detection of salmonella. J. Med. Microbiol. 2019, 68, 395–397. [Google Scholar] [CrossRef]
- Han, Z.; Shu, J.; Jiang, Q.; Cui, H. Coreactant-Free and Label-Free Eletrochemiluminescence Immunosensor for Copeptin Based on Luminescent Immuno-Gold Nanoassemblies. Anal. Chem. 2018, 90, 6064–6070. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, T. Rapid detection of Klebsiella pneumoniae, Klebsiella oxytoca, Raoultella ornithinolytica and other related bacteria in food by lateral-flow test strip immunoassays. J. Microbiol. Methods 2018, 147, 43–49. [Google Scholar] [CrossRef]
- Cheng, S.; Sun, J.; Yang, J.; Lv, J.; Wu, F.; Lin, Y.; Liao, L.; Ye, Y.; Cao, C.; Fang, L.; et al. A new immunoassay of serum antibodies against Peste des petits ruminants virus using quantum dots and a lateral-flow test strip. Anal. Bioanal. Chem. 2017, 409, 133–141. [Google Scholar] [CrossRef]
- Liu, B.; Si, J.; Zhao, F.; Wang, Q.; Wang, Y.; Li, J.; Li, C.; Li, T. Rapid detection of cow milk adulteration/contamination in goat milk by a lateral flow colloidal gold immunoassay strip. J. Dairy Res. 2019, 86, 94–97. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, Q.; Han, Q.; Chen, Q.; Tai, W.; Zhang, J.; Song, Y.; Xia, X. Detection of Shigella in Milk and Clinical Samples by Magnetic Immunocaptured-Loop-Mediated Isothermal Amplification Assay. Front. Microbiol. 2018, 9, 94. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Shi, Y.; Chen, C.; Han, Q.; Zhang, M.; Yi, H.; Song, Y.; Xia, X.; Zhang, J. Visual and Rapid Detection of Klebsiella pneumoniae by Magnetic Immunocapture-Loop-Mediated Isothermal Amplification Assay. Jundishapur. J. Microbiol. 2019, 12, e90016. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Y.; Chen, C.; Han, Q.; Chen, Q.; Xia, X.; Song, Y.; Zhang, J. Rapid, Visual Detection of Klebsiella pneumoniae Using Magnetic Nanoparticles and an Horseradish Peroxidase-Probe Based Immunosensor. J. Biomed. Nanotechnol. 2019, 15, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Yan, W.; Lijuan, L.; Dongxin, L.; Xia, L.; Yanmei, X.; Shoukui, H.; Lina, N.; Jianguo, X.; Changyun, Y. Rapid and Sensitive Detection of Shigella spp. and Salmonella spp. by Multiple Endonuclease Restriction Real-Time Loop-Mediated Isothermal Amplification Technique. Front. Microbiol. 2015, 6, 428–437. [Google Scholar] [CrossRef]
- Moussa, I.M.; Gassem, M.A.; Al-Doss, A.A.; Sadik, W.A.M.; Mawgood, A.A.L. Using molecular techniques for rapid detection of Salmonella serovars in frozen chicken and chicken products collected from Riyadh, Saudi Arabia. Afr. J. Biotechnol. 2010, 9, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Karp, B.E.; Campbell, D.; Chen, J.C.; Folster, J.P.; Friedman, C.R. Plasmid-mediated quinolone resistance in human non-typhoidal Salmonella infections: An emerging public health problem in the United States. Zoonoses Public Health 2018, 65, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Cahill, S.M.; Wachsmuth, I.K.; Costarrica Mde, L.; Ben Embarek, P.K. Powdered infant formula as a source of Salmonella infection in infants. Clin. Infect. Dis. 2008, 46, 268–273. [Google Scholar] [CrossRef]
- Amagliani, G.; Petruzzelli, A.; Carloni, E.; Tonucci, F.; Foglini, M.; Micci, E.; Ricci, M.; Di Lullo, S.; Rotundo, L.; Brandi, G. Presence of Escherichia coli O157, Salmonella spp., and Listeria monocytogenes in Raw Ovine Milk Destined for Cheese Production and Evaluation of the Equivalence Between the Analytical Methods Applied. Foodborne Pathog. Dis. 2016, 13, 626–632. [Google Scholar] [CrossRef]
- Kemal, J.; Sibhat, B.; Menkir, S.; Beyene, D. Prevalence, assessment, and antimicrobial resistance patterns of Salmonella from raw chicken eggs in Haramaya, Ethiopia. J. Infect. Dev. Ctries. 2016, 10, 1230–1235. [Google Scholar] [CrossRef] [Green Version]
- Nhung, N.T.; Van, N.T.B.; Cuong, N.V.; Duong, T.T.Q.; Nhat, T.T.; Hang, T.T.T.; Nhi, N.T.H.; Kiet, B.T.; Hien, V.B.; Ngoc, P.T.; et al. Antimicrobial residues and resistance against critically important antimicrobials in non-typhoidal Salmonella from meat sold at wet markets and supermarkets in Vietnam. Int. J. Food Microbiol. 2018, 266, 301–309. [Google Scholar] [CrossRef]
- Soria, M.C.; Soria, M.A.; Bueno, D.J.; Terzolo, H.R. Comparison of 3 culture methods and PCR assays for Salmonella gallinarum and Salmonella pullorum detection in poultry feed. Poult. Sci. 2013, 92, 1505–1515. [Google Scholar] [CrossRef]
- Yang, Y.G.; Song, M.K.; Park, S.J.; Kim, S.W. Direct detection of Shigella flexneri and Salmonella typhimurium in human feces by real-time PCR. J. Microbiol. Biotechnol. 2007, 17, 1616–1621. [Google Scholar] [PubMed]
- Warren, B.R.; Yuk, H.G.; Schneider, K.R. Detection of salmonella by flow-through immunocapture real-time PCR in selected foods within 8 hours. J. Food. Prot. 2007, 70, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jiang, X.; Qu, Y.; Pan, R.; Pang, X.; Jiang, Y.; Man, C. Salmonella detection in powdered dairy products using a novel molecular tool. J. Dairy. Sci. 2017, 100, 3480–3496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quigley, L.; O’Sullivan, O.; Beresford, T.P.; Paul Ross, R.; Fitzgerald, G.F.; Cotter, P.D. A comparison of methods used to extract bacterial DNA from raw milk and raw milk cheese. J. Appl. Microbiol. 2012, 113, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic. Acids. Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Notomi, T. Loop-mediated isothermal amplification (LAMP): A rapid, accurate, and cost-effective diagnostic method for infectious diseases. J. Infect. Chemother. 2009, 15, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Vaagt, F.; Haase, I.; Fischer, M. Loop-mediated isothermal amplification (LAMP)-based method for rapid mushroom species identification. J. Agric. Food. Chem. 2013, 61, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yamazaki, T.; Yo, A.; Tone, K.; Mahdi Alshahni, M.; Fujisaki, R.; Makimura, K. Detection of Fungi from an Indoor Environment using Loop-mediated Isothermal Amplification (LAMP) Method. Biocontrol. Sci. 2017, 22, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotiwan, N.; Brewster, C.D.; Magalhaes, T.; Weger-Lucarelli, J.; Duggal, N.K.; Ruckert, C.; Nguyen, C.; Garcia Luna, S.M.; Fauver, J.R. Rapid and specific detection of Asian- and African-lineage Zika viruses. Sci. Transl. Med. 2017, 9, e0538. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Dai, L.; Zhang, L.; Xu, K.; Song, Y.; Xia, X.; Han, Q.; Chen, Q.; Zhang, J. Efficient Capture and Detection of Zika Virion by Polyclonal Antibody Against Prokaryotic Recombinant Envelope Protein. Jundishapur. J. Microbiol. 2018, 11, e68858. [Google Scholar] [CrossRef]
- Chughtai, M.I.; Maqbool, U.; Iqbal, M.; Shah, M.S.; Fodey, T. Development of in-house ELISA for detection of chloramphenicol in bovine milk with subsequent confirmatory analysis by LC-MS/MS. J. Environ. Sci. Health B 2017, 52, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Sayeed, M.A.; Hossen, E.; Khanam, F.; Charles, R.C.; Andrews, J.; Ryan, E.T.; Qadri, F. Comparison of the Performance of the TPTest, Tubex, Typhidot and Widal Immunodiagnostic Assays and Blood Cultures in Detecting Patients with Typhoid Fever in Bangladesh, Including Using a Bayesian Latent Class Modeling Approach. PLoS Negl. Trop. Dis. 2016, 10, e0004558. [Google Scholar] [CrossRef] [PubMed]
- Bosqui, L.R.; Goncalves, A.L.R.; Goncalves-Pires, M.R.F.; Pavanelli, W.R.; Conchon-Costa, I.; Costa-Cruz, J.M.; Costa, I.N. Immune complex detection in saliva samples: An innovative proposal for the diagnosis of human strongyloidiasis. Parasitology 2018, 145, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Venkataramani, M.; Lakshminarayanan, H.; Saygili, E.; Moazeni, M.; Vasan, A.; Li, Y.; Sun, X.; Hua, S.; Yu, X.G.; et al. Nanoparticle-enhanced electrical detection of Zika virus on paper microchips. Nanoscale 2018, 10, 11841–11849. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Lakshminaraasimulu, N.K.; Krishnakumar, S.; Battalapalli, D.; Vasan, A.; Kanakasabapathy, M.K.; Sreeram, A.; Kallakuri, S.; Thirumalaraju, P.; Li, Y.; et al. Motion-Based Immunological Detection of Zika Virus Using Pt-Nanomotors and a Cellphone. ACS. Nano 2018, 12, 5709–5718. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Miks-Krajnik, M.; Yang, Y.; Lee, S.M.; Lee, S.C.; Yuk, H.G. Evaluation of real-time PCR coupled with immunomagnetic separation or centrifugation for the detection of healthy and sanitizer-injured Salmonella spp. on mung bean sprouts. Int. J. Food Microbiol. 2016, 222, 48–55. [Google Scholar] [CrossRef]
- Wolff, D.; Gerritzen, A. Overnight Enrichment is Essential for Reliable Salmonella PCR Testing from Fecal Samples. Clin. Lab. 2017, 63, 603–606. [Google Scholar] [CrossRef]
- Saeidabadi, M.S.; Nili, H.; Dadras, H.; Sharifiyazdi, H.; Connolly, J.; Valcanis, M.; Raidal, S.; Ghorashi, S.A. Evaluation of PCR and high-resolution melt curve analysis for differentiation of Salmonella isolates. Avian. Pathol. 2017, 46, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Chin, W.H.; Sun, Y.; Hogberg, J.; Hung, T.Q.; Wolff, A.; Bang, D.D. Solid-phase PCR for rapid multiplex detection of Salmonella spp. at the subspecies level, with amplification efficiency comparable to conventional PCR. Anal. Bioanal. Chem. 2017, 409, 2715–2726. [Google Scholar] [CrossRef]
- Yang, Q.; Domesle, K.J.; Wang, F.; Ge, B. Rapid detection of Salmonella in food and feed by coupling loop-mediated isothermal amplification with bioluminescent assay in real-time. BMC Microbiol. 2016, 16, 112. [Google Scholar] [CrossRef]
- Abdullah, J.; Saffie, N.; Sjasri, F.A.; Husin, A.; Abdul-Rahman, Z.; Ismail, A.; Aziah, I.; Mohamed, M. Rapid detection of Salmonella Typhi by loop-mediated isothermal amplification (LAMP) method. Braz. J. Microbiol. 2014, 45, 1385–1391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Wang, F.; Prinyawiwatkul, W.; Ge, B. Robustness of Salmonella loop-mediated isothermal amplification assays for food applications. J. Appl. Microbiol. 2014, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tatavarthy, A.; Ali, L.; Gill, V.; Hu, L.; Deng, X.; Adachi, Y.; Rand, H.; Hammack, T.; Zhang, G. Evaluation of Three Real-Time PCR Methods for Detection of Salmonella from Cloves. J. Food Prot. 2017, 80, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Biswas, S.; Aminabadi, P.; Stackhouse, J.W.; Jay-Russell, M.T.; Pandey, P.K. Prevalence of Escherichia coli O157 and Salmonella spp. in solid bovine manure in California using real-time quantitative PCR. Lett. Appl. Microbiol. 2019, 69, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.J.; Yang, Z.; Chen, X.B.; Tian, W.F.; Tu, C.N.; Wang, H.B. Verification and large scale clinical evaluation of a national standard protocol for Salmonella spp./Shigella spp. screening using real-time PCR combined with guided culture. J. Microbiol. Methods 2018, 145, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Rohonczy, K.; Zoller, L.; Hermann, Z.; Fodor, A.; Mraz, B.; Tabajdi-Pinter, V. Comparison of an automated ELFA and two different real-time PCR techniques for Salmonella detection in poultry samples. Acta Microbiol. Immunol. Hung. 2014, 61, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.B.; McDonough, P.L.; Anderson, R.R.; Franklin-Guild, R.J.; Ryan, J.R.; Perkins, G.A.; Thachil, A.J.; Glaser, A.L.; Thompson, B.S. Detection of Salmonella spp. in veterinary samples by combining selective enrichment and real-time PCR. J. Vet. Diagn. Invest. 2017, 29, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Volpe, G.; Delibato, E.; Fabiani, L.; Pucci, E.; Piermarini, S.; D’Angelo, A.; Capuano, F.; De Medici, D.; Palleschi, G. Development and evaluation of an ELIME assay to reveal the presence of Salmonella in irrigation water: Comparison with Real-Time PCR and the Standard Culture Method. Talanta 2016, 149, 202–210. [Google Scholar] [CrossRef]
- Almeida, C.; Cerqueira, L.; Azevedo, N.F.; Vieira, M.J. Detection of Salmonella enterica serovar Enteritidis using real time PCR, immunocapture assay, PNA FISH and standard culture methods in different types of food samples. Int. J. Food Microbiol. 2013, 161, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Yang, X.; Fang, Y.; Tong, Z.; Lin, H.; Fan, H. Detection of Salmonella Infection in Chickens by an Indirect Enzyme-Linked Immunosorbent Assay Based on Presence of PagC Antibodies in Sera. Foodborne Pathog. Dis. 2018, 15, 109–113. [Google Scholar] [CrossRef]
- Aribam, S.D.; Ogawa, Y.; Matsui, H.; Hirota, J.; Okamura, M.; Akiba, M.; Shimoji, Y.; Eguchi, M. Monoclonal antibody-based competitive enzyme-linked immunosorbent assay to detect antibodies to O:4 Salmonella in the sera of livestock and poultry. J. Microbiol. Methods 2015, 108, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lutze-Wallace, C.L.; Devenish, J.; Elmufti, M.; Burke, T. Development of an antigen-capture monoclonal antibody-based enzyme-linked immunosorbent assay and comparison with culture for detection of Salmonella enterica serovar Enteritidis in poultry hatchery environmental samples. J. Vet. Diagn. Invest. 2012, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, W.; Waldner, C.; Rajic, A.; McFall, M.; Chow, E.; Muckle, A. Comparison of bacterial culture, polymerase chain reaction, and a mix-enzyme-linked immunosorbent assay for the detection of Salmonella status in grow-to-finish pigs in western Canada with a Bayesian approach. Can. J. Vet. Res. 2011, 75, 308–311. [Google Scholar] [PubMed]
- Nyman, A.K.; Agren, E.C.; Bergstrom, K.; Wahlstrom, H. Evaluation of the specificity of three enzyme-linked immunosorbent assays for detection of antibodies against Salmonella in bovine bulk milk. Acta Vet. Scand. 2013, 55, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhu, Y.; Yin, K.; Xu, L.; Yin, C.; Li, Y.; Ren, J.; Yuan, Y.; Jiao, X. Purification of recombinant IpaJ to develop an indirect ELISA-based method for detecting Salmonella enterica serovar Pullorum infections in chickens. BMC. Vet. Res. 2019, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, R.; Wang, Y.; Wei, X.; Wang, Z.; Geng, Y.; Jing, J.; Gao, H.; Sun, X.; Dong, C.; et al. Development of duplex PCR-ELISA for simultaneous detection of Salmonella spp. and Escherichia coli O157: H7 in food. J. Microbiol. Methods 2018, 154, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Perelle, S.; Dilasser, F.; Malorny, B.; Grout, J.; Hoorfar, J.; Fach, P. Comparison of PCR-ELISA and LightCycler real-time PCR assays for detecting Salmonella spp. in milk and meat samples. Mol. Cell. Probes. 2004, 18, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Metzger-Boddien, C.; Bostel, A.; Kehle, J. AnDiaTec Salmonella sp. PCR-ELISA for analysis of food samples. J. Food Prot. 2004, 67, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, B.E.; Mathew, A.G.; Draughon, F.A.; Jayarao, B.M.; Oliveri, S.P. Detection of Salmonella enterica somatic groups C1 and E1 by PCR-enzyme-linked immunosorbent assay. J. Food Prot. 2003, 66, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Ravan, H.; Yazdanparast, R. Development and evaluation of a loop-mediated isothermal amplification method in conjunction with an enzyme-linked immunosorbent assay for specific detection of Salmonella serogroup D. Anal. Chim. Acta 2012, 733, 64–70. [Google Scholar] [CrossRef]
- Chinnappan, R.; AlAmer, S.; Eissa, S.; Rahamn, A.A.; Abu Salah, K.M.; Zourob, M. Fluorometric graphene oxide-based detection of Salmonella enteritis using a truncated DNA aptamer. Mikrochim. Acta 2017, 185, 61. [Google Scholar] [CrossRef] [PubMed]
- Bayrac, C.; Eyidogan, F.; Avni Oktem, H. DNA aptamer-based colorimetric detection platform for Salmonella Enteritidis. Biosens. Bioelectron. 2017, 98, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Jiang, Y.; Jia, F.; Yu, Y.; Chen, J.; Wang, Z. An aptamer-based electrochemical biosensor for the detection of Salmonella. J. Microbiol. Methods 2014, 98, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.R.; Sekhon, S.S.; Kim, S.G.; Rhee, S.J.; Yang, G.N.; Won, K.; Rhee, S.K.; Ryu, H.; Kim, K.; Min, J.; et al. Aptamer-Based Pathogen Monitoring for Salmonella enterica ser. Typhimurium. J. Biomed. Nanotechnol. 2018, 14, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, R.; Chen, F.; Jiang, T.; Wang, H.; Slavik, M.; Wei, H.; Li, Y. QCM-based aptamer selection and detection of Salmonella typhimurium. Food Chem. 2017, 221, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Li, Z.; Luo, F.; Ai, S.; Chen, B.; Wang, Q. Electrochemical determination of Salmonella typhimurium by using aptamer-loaded gold nanoparticles and a composite prepared from a metal-organic framework (type UiO-67) and graphene. Mikrochim. Acta 2019, 186, 620. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.H.; Lee, B.; Kim, D.; Lee, J.K.; Kim, S.; Bae, J.; Park, Y.; Lee, K. Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar Typhimurium. Biomaterials 2016, 104, 43–51. [Google Scholar] [CrossRef]
- Xu, X.; Ma, X.; Wang, H.; Wang, Z. Aptamer based SERS detection of Salmonella typhimurium using DNA-assembled gold nanodimers. Mikrochim. Acta 2018, 185, 325. [Google Scholar] [CrossRef]
- Zhang, L.; Du, X.; Chen, C.; Chen, Z.; Zhang, L.; Han, Q.; Xia, X.; Song, Y.; Zhang, J. Development and Characterization of Double-Antibody Sandwich ELISA for Detection of Zika Virus Infection. Viruses 2018, 10, 634. [Google Scholar] [CrossRef]








| Results | Methods | |||||||
|---|---|---|---|---|---|---|---|---|
| AIB | PCR [13,15,47,48,49] | LAMP [9,14,50,51,52] | Real-Time PCR [31,32,46,53,54,55,56] | IC-PCR [46,57,58,59] | ELISA [60,61,62,63,64,65] | PCR [66,67,68,69], LAMP-ELISA [70] | DNA Aptamer Assay [71,72,73,74,75,76,77,78] | |
| Sensitivity | 9 CFU for artificial sample | 102 to105 CFU for artificial sample | 1.3 to 28 CFU for artificial sample | 102 to 104 CFU for artificial sample | 102 to 103 CFU for artificial sample | 102 to 103 CFU for artificial sample | 101 to 103 CFU for artificial sample | 101 to 103 CFU for artificial sample |
| Need times | 50 min | 14 h | 3 h | 1 h to 8 h | 1.5 h | 8 h to 23 h | 8 h to 23 h | 3 h to 23 h |
| Equipment | Magnet, TMB buffer | Bacterial enrichment, genomic extraction kit, PCR equipment and related reagents, DNeasy | Bacterial enrichment, genomic extraction kit, LAMP equipment and related reagents | Bacterial enrichment, genomic extraction kit, real-time PCR equipment and related reagents | Magnet, genomic extraction kit, real-time PCR equipment and related reagents | 96-well plates, antibodies, PBS-T, TMB buffer | PCR or LAMP equipment and related reagents, 96-well plates, antibodies, PBS-T, TMB buffer | Aptamers, PBS-T, PBS, centrifuge, TMB buffer |
| Bacterial | Bacterial Strains Source |
|---|---|
| Salmonella | Isolate from monkey |
| Salmonella | ATCC13076 |
| Shigella | Isolate from monkey |
| A. baumannii | Isolate from clinical samples |
| P. aeruginosa | Isolate from secretion substance |
| E. coli | ATCC25922 |
| K. pneumoniae | Isolate from clinical samples |
| S. aureus | ATCC29213 |
| Streptococcus | Isolate from clinical samples |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Du, X.; Chen, Z.; Chen, C.; Gong, N.; Song, Y.; Song, Y.; Han, Q.; Xia, X.; Luo, H.; et al. Instrument-Free and Visual Detection of Salmonella Based on Magnetic Nanoparticles and an Antibody Probe Immunosensor. Int. J. Mol. Sci. 2019, 20, 4645. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184645
Zhang L, Du X, Chen Z, Chen C, Gong N, Song Y, Song Y, Han Q, Xia X, Luo H, et al. Instrument-Free and Visual Detection of Salmonella Based on Magnetic Nanoparticles and an Antibody Probe Immunosensor. International Journal of Molecular Sciences. 2019; 20(18):4645. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184645
Chicago/Turabian StyleZhang, Liding, Xuewei Du, Zhixin Chen, Congjie Chen, Nanxin Gong, Yihao Song, Yuzhu Song, Qinqin Han, Xueshan Xia, Haiming Luo, and et al. 2019. "Instrument-Free and Visual Detection of Salmonella Based on Magnetic Nanoparticles and an Antibody Probe Immunosensor" International Journal of Molecular Sciences 20, no. 18: 4645. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184645