Hydroxyapatite Formation on Self-Assembling Peptides with Differing Secondary Structures and Their Selective Adsorption for Proteins
Abstract
:1. Introduction
2. Results and Discussion
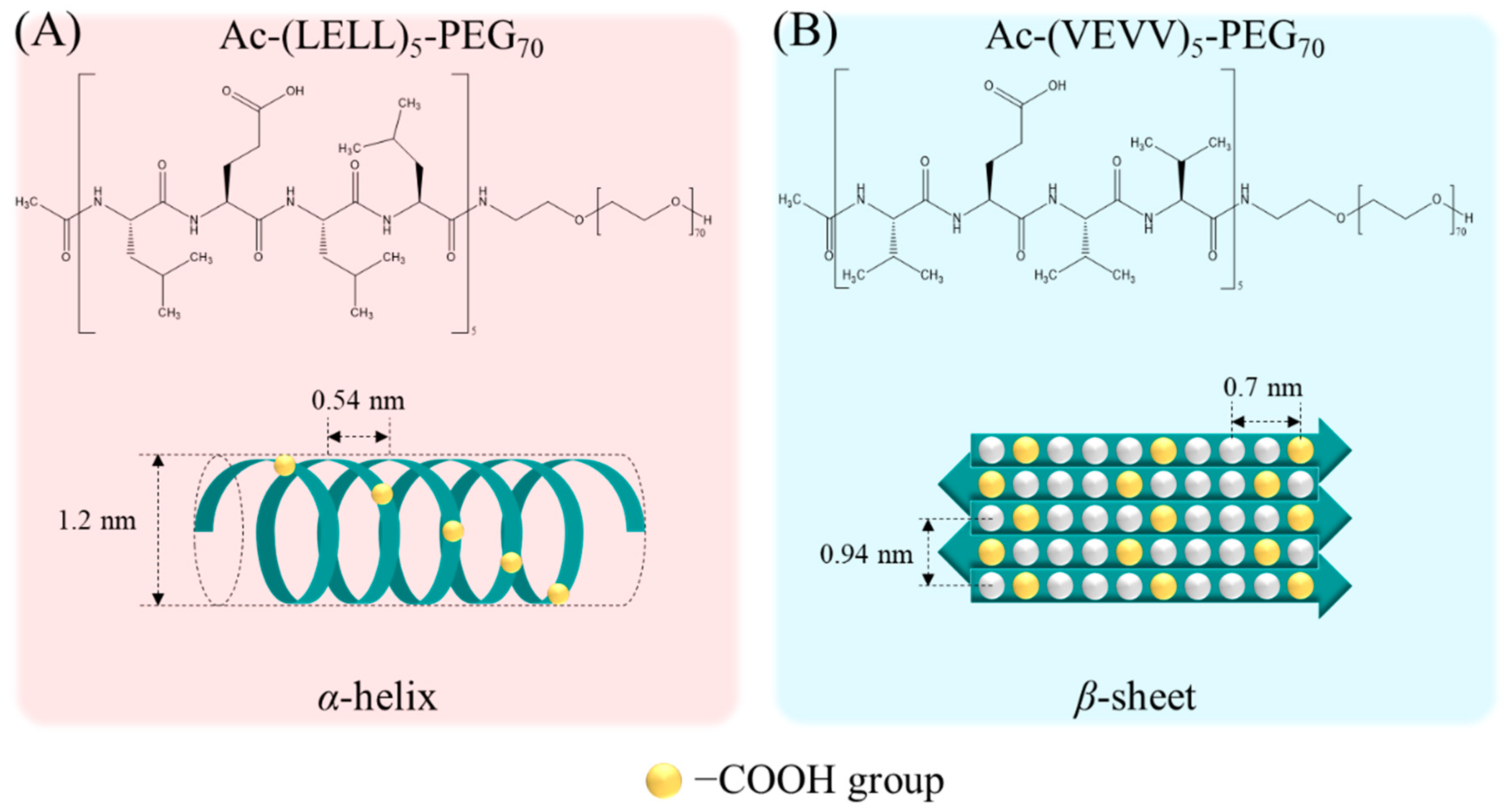
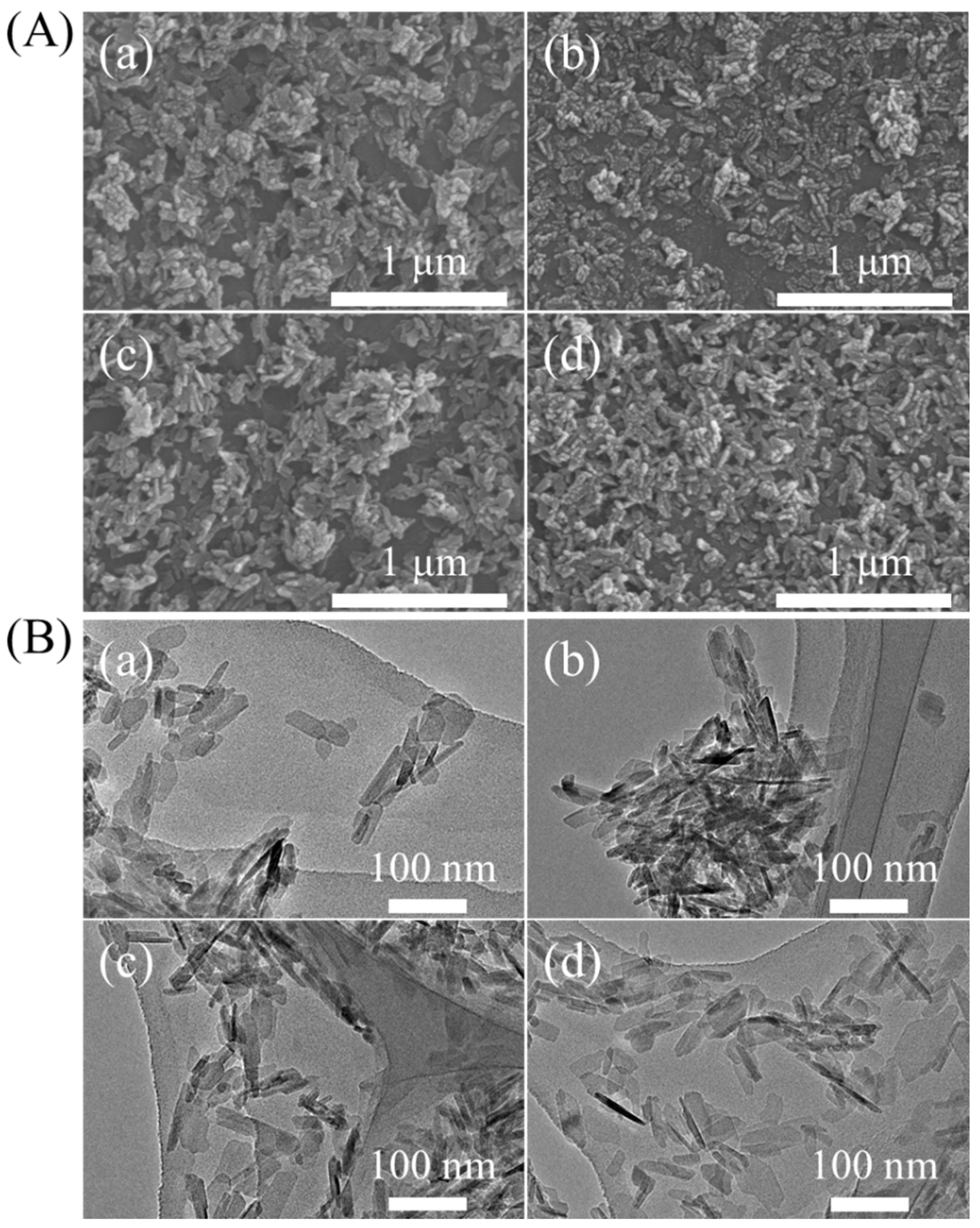
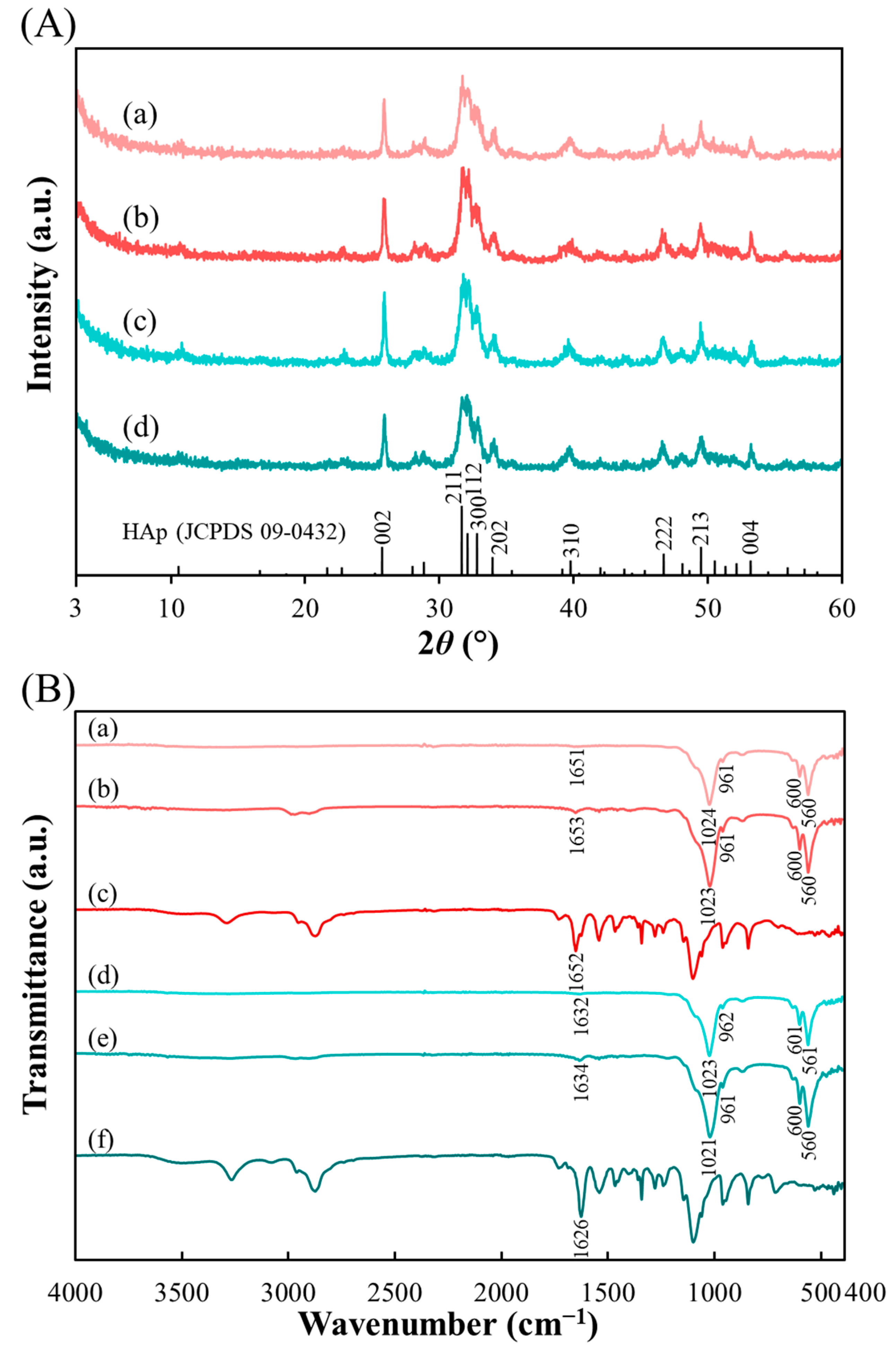
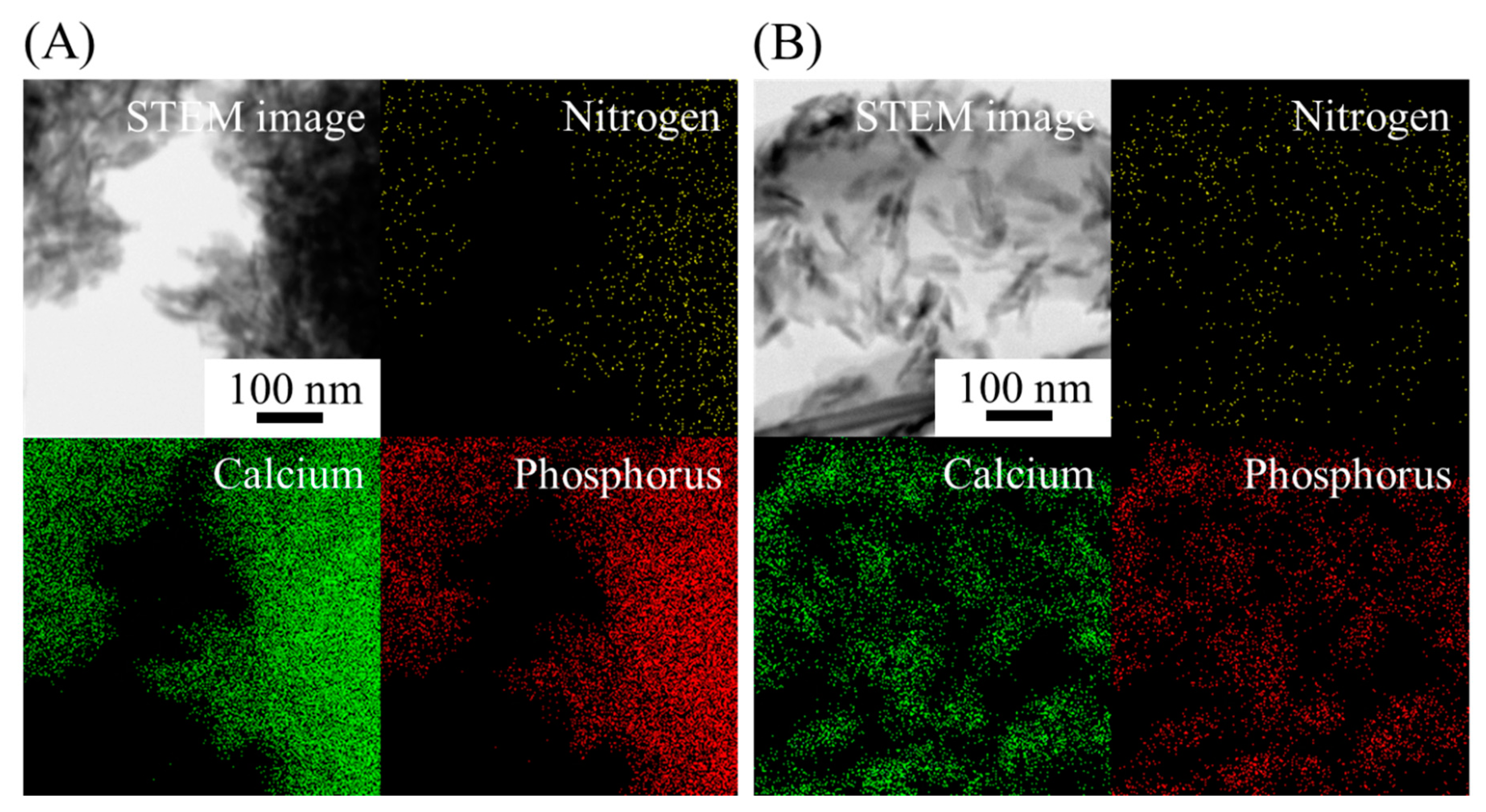
2.1. Peptide–HAp Characterization
2.2. Protein Adsorption on Peptide–HAp
2.3. Carboxyl Group Density in Peptide–HAp
3. Materials and Methods
3.1. Materials
3.2. Preparation of Peptide–HAp Particles
3.3. Characterization of Synthesized Peptide–HAp
3.4. Protein Adsorption on Peptide–HAp
3.5. Calculation of Carboxyl Group Density in Peptide–HAp
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HAp | Hydroxyapatite |
| PEG | Poly(ethylene glycol) |
| CD | Circular dichroism |
| FE-SEM | Field-emission scanning electron microscopy |
| TEM | Transmission electron microscopy |
| BET | Brunauer-Emmett-Teller |
| BJH | Barrett-Joyner-Halenda |
| XRD | Powder X-ray diffraction |
| TG-DTA | Thermogravimetry and differential thermal analysis |
| FTIR | Fourier transform infrared |
| STEM | Scanning transmission electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| Cyt c | Cytochrome c |
| MGB | Myoglobin |
| BSA | Bovine serum albumin |
| LSZ | Lysozyme |
| OVT | Conalbumin |
| TF | Transferrin |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| NHS | N-Hydroxysuccinimide |
References
- Jiang, W.; Yi, X.; McKee, M.D. Chiral biomineralized structures and their biomimetic synthesis. Mater. Horiz. 2019. [Google Scholar] [CrossRef]
- Eichler-Volf, A.; Xue, L.; Dornberg, G.; Chen, H.; Kovalev, A.; Enke, D.; Wang, Y.; Gorb, E.V.; Gorb, S.N.; Steinhart, M. The influence of surface topography and surface chemistry on the anti-adhesive performance of nanoporous monoliths. ACS. Appl. Mater. Interfaces 2016, 8, 22593–22604. [Google Scholar] [CrossRef]
- Cui, Y.; Li, D.; Bai, H. Bioinspired smart materials for directional liquid transport. Ind. Eng. Chem. Res. 2017, 56, 4887–4897. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Liu, X.; Liu, J.; Lu, G.; Kaplan, D.L.; Zhu, H.; Lu, Q. Biomineralization of stable and monodisperse vaterite microspheres using silk nanoparticles. ACS. Appl. Mater. Interfaces 2015, 7, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yang, Y.; Cao, Y.; Song, Y.; Xu, L.; Zhang, X.; Wang, S. Bioinspired DNA-inorganic hybrid nanoflowers combined with a personal glucose meter for onsite detection of miRNA. ACS. Appl. Mater. Interfaces 2018, 10, 42050–42057. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, Y.; Munyemana, J.C.; Wu, T.; Yang, Z.; Chen, H.; Qu, W.; Xiao, J. Tuning the hierarchical nanostructure of hematite mesocrystals via collagen-templated biomineralization. J. Mater. Chem. B 2017, 5, 1423–1429. [Google Scholar] [CrossRef]
- Galloway, J.M.; Staniland, S.S. Protein and peptide biotemplated metal and metal oxide nanoparticles and their pattering onto surfaces. J. Mater. Chem. 2012, 22, 12423–12434. [Google Scholar] [CrossRef]
- Chen, C.; Rosi, N.L. Peptide-based methods for the preparation of nanostructured inorganic materials. Angew. Chem. Int. Ed. 2010, 49, 1924–1942. [Google Scholar] [CrossRef]
- Dickerson, M.B.; Sandhage, K.H.; Naik, R.R. Protein- and peptide-directed synthesis of inorganic materials. Chem. Rev. 2008, 108, 4935–4978. [Google Scholar] [CrossRef]
- Sethi, M.; Pacardo, D.B.; Knecht, M.R. Biological surface effects of metallic nanomaterials for applications in assembly and catalysis. Langmuir 2010, 26, 15121–15134. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Wada, N.; Horiuchi, N.; Nakamura, M.; Nozaki, K.; Nagai, A.; Yamashita, K. Controlled crystallization of calcium carbonate via cooperation of polyaspartic acid and polylysine under double-diffusion conditions in agar hydrogels. ACS Omega 2018, 3, 16681–16692. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Chen, C.; Chang, Y. Biomimetic synthesis of silica films directed by polypeptide brushes. Chem. Mater. 2008, 20, 6148–6156. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, W.; Zhu, S.; Yu, X.; Yan, D. Novel morphology of calcium carbonate controlled by poly(l-lysine). Langmuir 2009, 25, 13238–13243. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Lin, J.; Lu, Y.; Zhang, Q.; Wang, L. Polypeptide self-assemblies: Nanostructures and bioapplications. Chem. Soc. Rev. 2016, 45, 5985–6012. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A.S.; Koch, M.; Guth, C.; Weiss, I.M. Peptide induced crystallization of calcium carbonate on wrinkle patterned substrate: Implications for chitin formation in Molluscs. Int. J. Mol. Sci. 2013, 14, 11842–11860. [Google Scholar] [CrossRef]
- Bhandari, R.; Pacardo, D.B.; Bedford, N.M.; Naik, R.R.; Knecht, M.R. Structural control and catalytic reactivity of peptide-templated Pd and Pt nanomaterials for olefin hydrogenation. J. Phys. Chem. C 2013, 117, 18053–18062. [Google Scholar] [CrossRef]
- Yuwono, V.M.; Hartgerink, J.D. Peptide amphiphile nanofibers template and catalyze silica nanotube formation. Langmuir 2007, 23, 5033–5038. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Liu, R.; Sun, Z.; Wei, Y.; Zhao, Y.; Gao, X. Serial silver clusters biomineralized by one peptide. ACS Nano 2011, 5, 8684–8689. [Google Scholar] [CrossRef]
- Yildirim, A.; Acar, H.; Erkal, T.S.; Bayindir, M.; Guler, M.O. Template-directed synthesis of silica nanotubes for explosive detection. ACS. Appl. Mater. Interfaces 2011, 3, 4159–4164. [Google Scholar] [CrossRef]
- Acar, H.; Garifullin, R.; Guler, M.O. Self-assembled template-directed synthesis of one-dimensional silica and titania nanostructures. Langmuir 2011, 27, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Susapto, H.H.; Kudu, O.U.; Garifullin, R.; Yilmaz, E.; Guler, M.O. One-dimensional peptide nanostructure templated growth of iron phosphate nanostructures for lithium-ion battery cathodes. ACS. Appl. Mater. Interfaces 2016, 8, 17421–17427. [Google Scholar] [CrossRef] [PubMed]
- Tomizaki, K.; Kubo, S.; Ahn, S.; Satake, M.; Imai, T. Biomimetic alignment of zinc oxide nanoparticles along a peptide nanofiber. Langmuir 2012, 28, 13459–13466. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Thomas, K.G. Surface plasmon coupled circular dichroism of Au nanoparticles on peptide nanotubes. J. Am. Chem. Soc. 2010, 132, 2502–2503. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Schäfer, A.; Lutz, H.; Roeters, S.J.; Lieberwirth, I.; Muñoz-Espí, R.; Hood, M.A.; Bonn, M.; Weidner, T. Peptide-controlled assembly of macroscopic calcium oxalate nanosheets. J. Phys. Chem. Lett. 2019, 10, 2170–2174. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Wang, J.; Li, Y.; Xia, D.; Shan, H.; Xu, H.; Lu, J.R. Short peptide-directed synthesis of one-dimensional platinum nanostructures with controllable morphologies. Sci. Rep. 2013, 3, 2565–2570. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xue, J.; Zhao, Y.; Du, M.; Deng, L.; Xu, H.; Lu, J.R. Controlled silica deposition on self-assembled peptide nanostructures via varying molecular structures of short amphiphilic peptides. Soft Matter 2014, 10, 7623–7629. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cai, Q.; Du, M.; Xue, J.; Xu, H. Synthesis of 1D silica nanostructures with controllable sizes based on short anionic peptide self-assembly. J. Phys. Chem. B 2015, 119, 12059–12065. [Google Scholar] [CrossRef]
- Du, M.; Bu, Y.; Zhou, Y.; Zhao, Y.; Wang, S.; Xu, H. Peptide-templated synthesis of branched MnO2 nanowires with improved electrochemical performances. RSC Adv. 2017, 7, 12711–12718. [Google Scholar] [CrossRef]
- Nonoyama, T.; Kinoshita, T.; Higuchi, M.; Nagata, K.; Tanaka, M.; Sato, K.; Kato, K. Multistep growth mechanism of calcium phosphate in the earliest stage of morphology-controlled biomineralization. Langmuir 2011, 27, 7077–7083. [Google Scholar] [CrossRef]
- Kuno, T.; Nonoyama, T.; Hirao, K.; Kato, K. Influence of the charge relay effect on the silanol condensation reaction as a model for silica biomineralization. Langmuir 2011, 27, 13154–13158. [Google Scholar] [CrossRef] [PubMed]
- Nonoyama, T.; Kinoshita, T.; Higuchi, M.; Nagata, K.; Tanaka, M.; Sato, K.; Kato, K. TiO2 synthesis inspired by biomineralization: Control of morphology, crystal phase, and light-use efficiency in a single process. J. Am. Chem. Soc. 2012, 134, 8841–8847. [Google Scholar] [CrossRef] [PubMed]
- Murai, K.; Higuchi, M.; Kinoshita, T.; Nagata, K.; Kato, K. Calcium carbonate biomineralization utilizing a multifunctional β-sheet peptide template. Chem. Commun. 2013, 49, 9947–9949. [Google Scholar] [CrossRef] [PubMed]
- Morejón, L.; Delgado, J.A.; Ribeiro, A.A.; Oliveira, M.V.; Mendizábal, E.; García, I.; Alfonso, A.; Poh, P.; Griensven, M.; Balmayor, E.R. Development, characterization and in vitro biological properties of scaffolds fabricated from calcium phosphate nanoparticles. Int. J. Mol. Sci. 2019, 20, 1790. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Yang, J.; Dai, J.; Qin, Y.; Wang, Y.; Guo, Y.; Ke, Q.; Zhang, C. Bioinspired nanostructured hydroxyapatite/collagen three-dimensional porous scaffolds for bone tissue engineering. RSC Adv. 2015, 5, 36175–36184. [Google Scholar] [CrossRef]
- Cai, Y.; Li, H.; Karlsson, M.; Leifer, K.; Engqvist, H.; Xia, W. Biomineralization on single crystalline rutile: The modulated growth of hydroxyapatite by fibronectin in a simulated body fluid. RSC Adv. 2016, 6, 35507–35516. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Zhang, Q.; Liu, W.; Liu, Y.; Banks, C.E. Multi-dimensional hydroxyapatite (HAp) nanocluster architectures fabricated via nafion-assisted biomineralization. New J. Chem. 2015, 39, 750–754. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, Q.; Sahai, N. How does bone sialoprotein promote the nucleation of hydroxyapatite? A molecular dynamics study using model peptides of different conformations. Langmuir 2010, 26, 9848–9859. [Google Scholar] [CrossRef] [PubMed]
- Weiger, M.C.; Park, J.J.; Roy, M.D.; Stafford, C.M.; Karim, A.; Becker, M.L. Quantification of the binding affinity of a specific hydroxyapatite binding peptide. Biomaterials 2010, 31, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Ruan, Q.; Nutt, S.; Tao, J.; Yoreo, J.J.D.; Moradian-Oldak, J. Peptide-based bioinspired approach to regrowing multilayered aprismatic enamel. ACS Omega 2018, 3, 2546–2557. [Google Scholar] [CrossRef]
- Hadagalli, K.; Panda, A.K.; Mandal, S.; Basu, B. Faster biomineralization and tailored mechanical properties of marine-resource-derived hydroxyapatite scaffolds with tunable interconnected porous architecture. ACS Appl. Bio Mater. 2019, 2, 2171–2184. [Google Scholar] [CrossRef]
- Wei, P.; Yuan, Z.; Jing, W.; Huang, Y.; Cai, Q.; Guan, B.; Liu, Z.; Zhang, X.; Mao, J.; Chen, D.; et al. Strengthening the potential of biomineralized microspheres in enhancing osteogenesis via incorporating alendronate. Chem. Eng. J. 2019, 368, 577–588. [Google Scholar] [CrossRef]
- Kojima, S.; Nagata, F.; Kugimiya, S.; Kato, K. Synthesis of peptide-containing calcium phosphate nanoparticles exhibiting highly selective adsorption of various proteins. Appl. Surf. Sci. 2018, 458, 438–445. [Google Scholar] [CrossRef]
- Kojima, S.; Nagata, F.; Inagaki, M.; Kugimiya, S.; Kato, K. Avidin-adsorbed peptide–calcium phosphate composites exhibiting high biotin-binding activity. New J. Chem. 2019, 43, 427–435. [Google Scholar] [CrossRef]
- Kojima, S.; Nagata, F.; Inagaki, M.; Kugimiya, S.; Kato, K. Enzyme immobilisation on poly-L-lysine-containing calcium phosphate particles for highly sensitive glucose detection. RSC Adv. 2019, 9, 10832–10841. [Google Scholar] [CrossRef]
- Kuno, T.; Nonoyama, T.; Hirao, K.; Kato, K. Structural formation ability of peptide secondary structure on silica biomineralization. Chem. Lett. 2012, 41, 1547–1549. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, H.; Peng, M.; Lei, Q.; Zhuo, R.; Zhang, X. Adjustable nanofibers self-assembled from an irregular conformational peptide amphiphile. Polym. Chem. 2015, 6, 519–524. [Google Scholar] [CrossRef]
- Fry, H.C.; Silveira, G.Q.; Cohn, H.M.; Lee, B. Diverse bilayer morphologies achieved via α-helix-to-β-sheet transitions in a short amphiphilic peptide. Langmuir 2019, 35, 8961–8967. [Google Scholar] [CrossRef]
- Stevanović, M.; Ðošić, M.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Stojanović, J.; Odović, J.; Sakač, M.C.; Rhee, K.Y.; Mišković-Stanković, V. Gentamicin-loaded bioactive hydroxyapatite/chitosan composite coating electrodeposited on titanium. ACS Biomater. Sci. Eng. 2018, 4, 3994–4007. [Google Scholar] [CrossRef]
- Suades, A.; Alcaraz, A.; Cruz, E.; Álvarez-Marimon, E.; Whitelegge, J.P.; Manyosa, J.; Cladera, J.; Perálvarez-Marín, A. Structural biology workflow for the expression and characterization of functional human sodium glucose transporter type 1 in Pichia pastoris. Sci. Rep. 2019, 9, 1203–1213. [Google Scholar] [CrossRef]



| Sample | Surface Area (a) (m2 g−1) | Pore Volume (a) (cm3 g−1) | Ca/P Molar Ratio (b) | Amount of Peptide (c) (mg) | Zeta Potential (d) (mV) |
|---|---|---|---|---|---|
| LELL–HAp (1 mg) | 106 | 0.81 | 1.52 | 0.43 | −19.8 |
| LELL–HAp (3 mg) | 101 | 0.71 | 1.50 | 1.4 | −12.8 |
| VEVV–HAp (1 mg) | 101 | 0.64 | 1.52 | 0.51 | −12.5 |
| VEVV–HAp (3 mg) | 92 | 0.62 | 1.51 | 1.5 | −11.1 |
| Sample | α-Helix | β-Sheet | β-Turn | Other |
|---|---|---|---|---|
| LELL | 94% | — * | 3% | 3% |
| LELL + Ca (a) | >99% | — * | — * | — * |
| VEVV | — * | 96% | 1% | 3% |
| VEVV + Ca (a) | 2% | 47% | 24% | 27% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, S.; Nakamura, H.; Lee, S.; Nagata, F.; Kato, K. Hydroxyapatite Formation on Self-Assembling Peptides with Differing Secondary Structures and Their Selective Adsorption for Proteins. Int. J. Mol. Sci. 2019, 20, 4650. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184650
Kojima S, Nakamura H, Lee S, Nagata F, Kato K. Hydroxyapatite Formation on Self-Assembling Peptides with Differing Secondary Structures and Their Selective Adsorption for Proteins. International Journal of Molecular Sciences. 2019; 20(18):4650. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184650
Chicago/Turabian StyleKojima, Suzuka, Hitomi Nakamura, Sungho Lee, Fukue Nagata, and Katsuya Kato. 2019. "Hydroxyapatite Formation on Self-Assembling Peptides with Differing Secondary Structures and Their Selective Adsorption for Proteins" International Journal of Molecular Sciences 20, no. 18: 4650. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20184650