Unravelling the Metabolic and Hormonal Machinery During Key Steps of Somatic Embryogenesis: A Case Study in Coffee
Abstract
:1. Introduction
2. Results
2.1. Morphological and Histological Characterization of Arabica SE Key Sampled Stages
2.2. Clustering Metabolite Profiles Divides the Arabica SE Process into Five Major Phases
2.3. Metabolic Characterization of the Five Phases of the Arabica SE Process
2.4. Cell Hormonal Content Changes throughout the Arabica SE Process
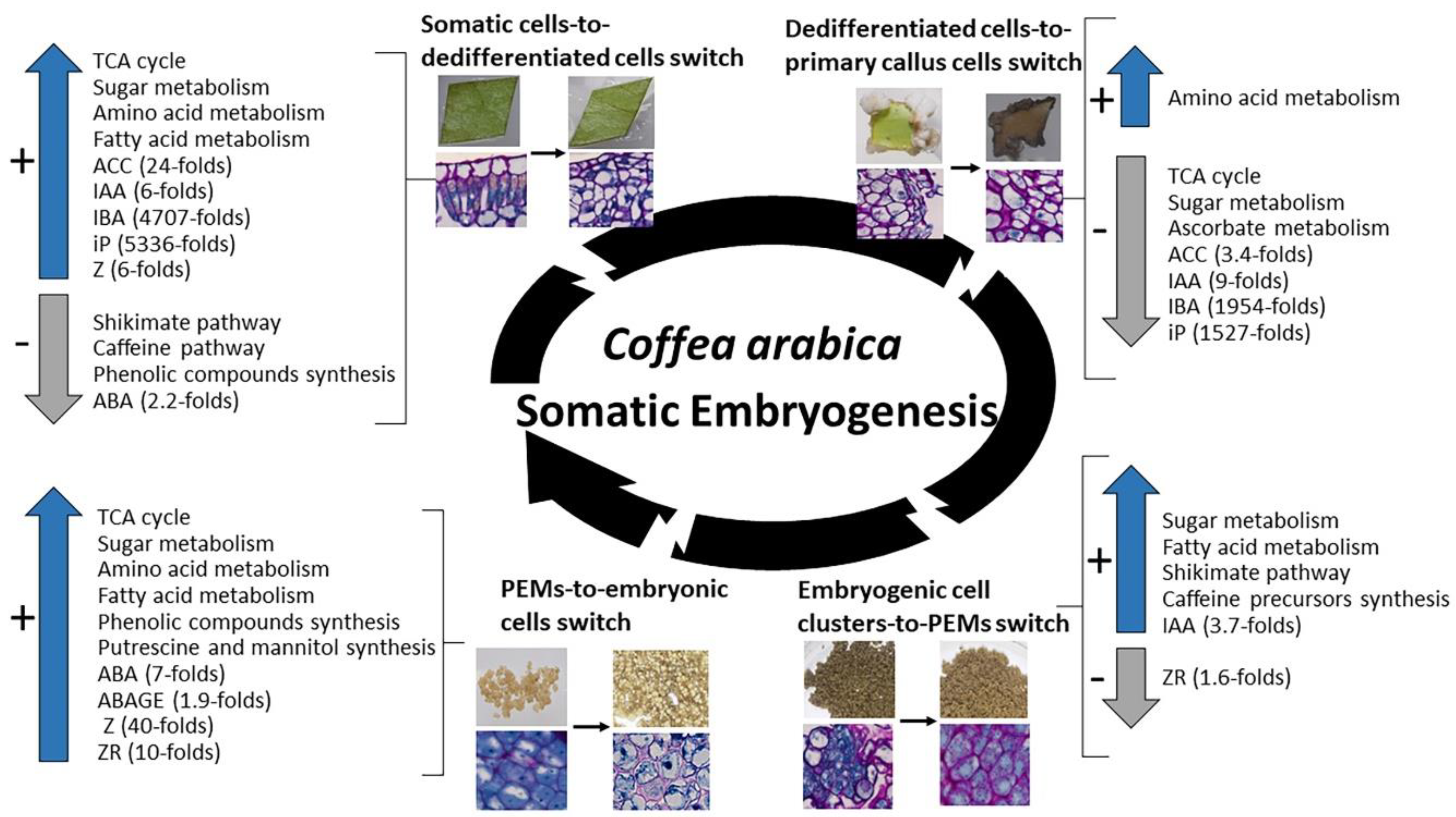
2.5. Most Significant Changes in Metabolic and Hormonal Profiles Occur during Phase Switches
- (i)
- The leaf explant-to-dedifferentiated cell switch occurring after one week in induction medium (L2 stage to D1). During this switch, and even though no change could be observed on a morphological basis, huge changes were noticed at a biochemical level. Our results show a drastic increase in levels of metabolites involved in the TCA cycle pathway, in sugar (glucose-6-phosphate, fructose-6-phosphate, mannose-6-phosphate), amino acid and fatty acid metabolisms while shikimate, caffeine and phenolic compounds pathways were poorly present. Hormone levels also sharply increased during this phase switch mainly ACC (24-fold), IAA (6-fold), IBA (4707-fold), iP (5336-fold) and Z (6-fold), except for ABA which levels were decreased (2.2-fold).
- (ii)
- The dedifferentiated cells-to-established primary callus cells switch occurring three months after induction (D3 stage to C1). This switch was characterized by a sharp decrease in cell basic functions, mainly a decrease in the levels of metabolites involved in the TCA cycle pathway, sugar metabolism (phosphorylated glucose and fructose), and the ascorbate pathway which is related to the cell photosynthetic activity. Only synthesis of some amino acids was maintained (methionine, glycine). Hormone levels also sharply decreased in compact primary callus cells compared to cells in early dedifferentiation mainly IBA (1954-folds), iP (1527-fold), IAA (9-fold) and ACC (3.4-fold).
- (iii)
- The embryogenic cell clusters-to-PEMs switch occurring after one week in redifferentiation medium (C3 stage to R1). During this switch, a re-activation in cell primary and secondary functions was observed; levels of phosphorylated sugars (glucose-6-phosphate, fructose-6-phosphate, mannose-6-phosphate) and fatty acids (glycerol-3-phosphate) increased as well as precursors of caffeine (inosine, xanthosine) and end-products of the shikimate pathway (caffeic acid, benzoic acid, tryptophan). In parallel, IAA levels also increased in PEMs compared to embryogenic cell clusters (3.7-fold) while levels of ZR sharply decreased (1.6-fold).
- (iv)
- The PEMs-to-embryonic cells switch occurring after four weeks in redifferentiation medium (R4 stage to E1). During this switch, cell functions are re-established in embryonic cells with increased levels of TCA cycle metabolites, phosphorylated sugars and fatty acids, and the synthesis of a number of amino acids (alanine, glycine, leucine, lysine, methionine, proline, glutamate) and phenolic compounds (5-CQA; 3-CQA; 4-CQA; 3,5-di-CQA; 4,5-diCQA and FQA). A synthesis of putrescine and mannitol was also noticed. Our results also showed a significant increase in ABA levels (7-fold), ABAGE levels (1.9-fold), Z levels (40-fold) and ZR levels (40-fold) in embryonic cells compared to PEMs.
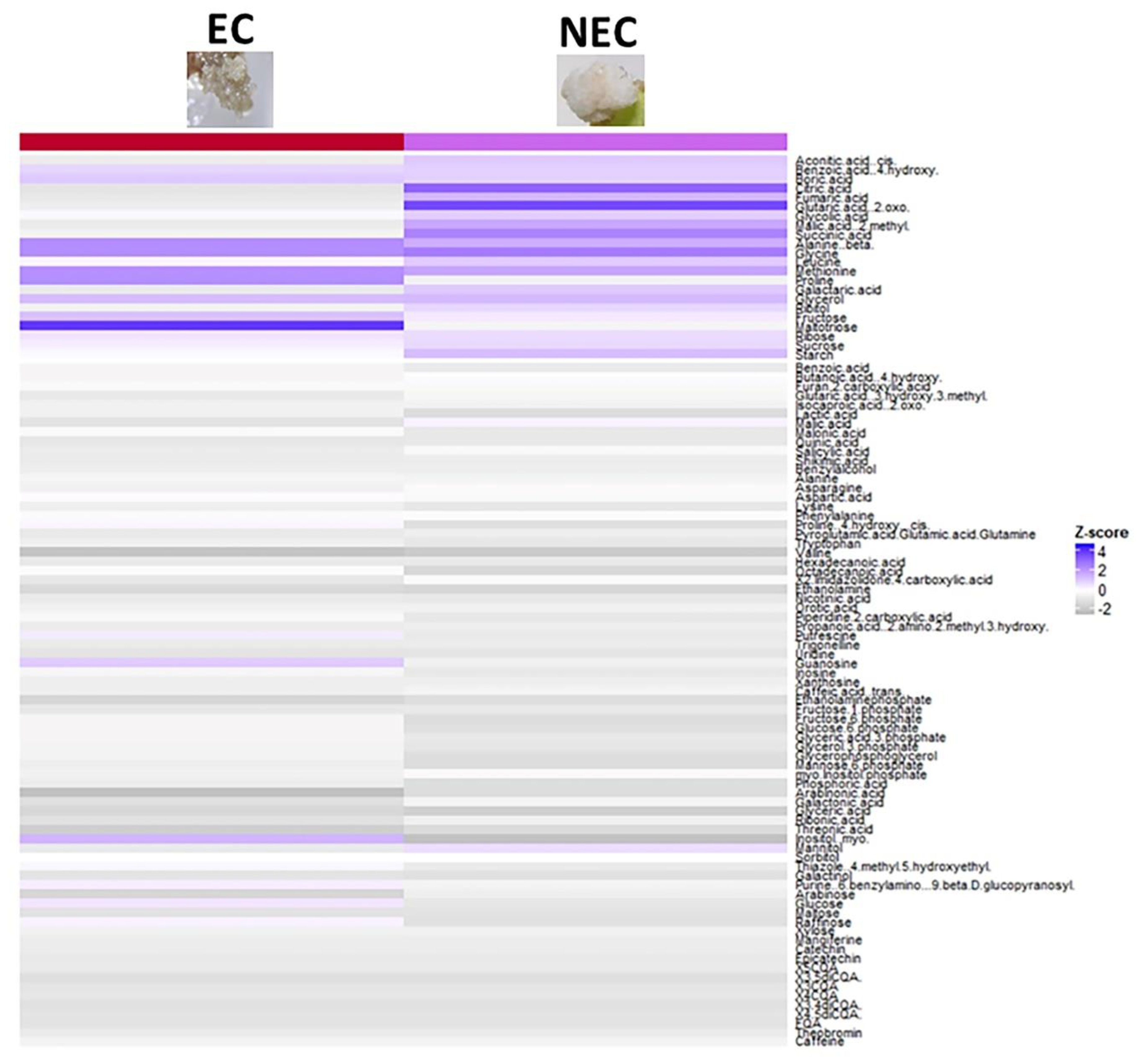
2.6. Embryogenic and Non-embryogenic Calli Differ Morphologically, Metabolically and Hormonally
3. Discussion
3.1. Arabica SE Metabolites as a Signature of Cell Fate
3.2. The Dedifferentiation Episode Is Characterized by Huge Changes in Cell Metabolic Pathways
3.3. Identifying Metabolic Markers of the Embryogenic Capacity
3.4. Metabolic and Hormonal Profiles of the Callus Phase Are Proof of Cell Totipotency
3.5. Re-Establishment of Primary and Secondary Metabolims During Redifferentiation Leading to Embryo Development
4. Materials and Methods
4.1. Tissue Culture and Sampling
4.2. Primary Metabolite Analysis
4.3. Secondary Metabolite Analysis
4.4. Hormone Content Analysis
4.5. Starch Content Assay
4.6. Histological Analysis and Cell Imaging
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| 2-iP | N6-(2-Isopentenyl) adenine |
| 6-BA | 6-Benzylaminopurine |
| ABA | cis-Abscisic acid |
| ABAGE | ABA glucose ester |
| ACC | 1-Aminocyclopropane-1-carboxylic acid |
| BSTFA | N,O-Bis(trimethylsilyl)trifluoroacetamide |
| CQA | Caffeoylquinic acid |
| DAD | Photodiode array detector |
| DW | Dry weight |
| EC | Embryogenic callus |
| FQA | Feruloylquinic acid |
| GC–EI–TOF/MS | Gas chromatography-electron impact ionization-time of flight/mass spectrometry |
| GC-MS | Gas chromatography–mass spectrometry |
| HPLC | High-performance liquid chromatography |
| IAA | Indole-3-acetic acid |
| IBA | Indole 3-butyric acid |
| iP | Isopentenyladenine |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS | Liquid chromatography–mass spectrometry |
| MeOH | Methanol |
| MS | Murashige and Skoog |
| N/C ratio | Nucleocytoplasmic ratio |
| NEC | Non-embryogenic callus |
| PCA | Principal Component Analysis |
| PEMs | Pro-embryogenic masses |
| SE | Somatic embryogenesis |
| TCA | Tricarboxylic acid |
| UPLC-ESI–MS/MS | Ultra-performance liquid chromatography-electrospray tandem mass spectrometry |
| W/L ratio | Width-to-length ratio |
| Z | Zeatin (cis- and trans-) |
| ZR | Zeatin riboside (cis- and trans-) |
References
- Verdeil, J.-L.; Alemanno, L.; Niemenak, N.; Tranbarger, T.J. Pluripotent versus totipotent plant stem cells: Dependence versus autonomy? Trends Plant Sci. 2007, 12, 245–252. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Harashima, H.; Shibata, M.; Ohnuma, M.; Breuer, C.; Morao, A.K.; de Lucas, M.; De Veylder, L.; et al. PRC2 represses dedifferentiation of mature somatic cells in Arabidopsis. Nat. Plants 2015, 1, 15089. [Google Scholar] [CrossRef]
- Zeng, F.; Zhang, X.; Jin, S.; Cheng, L.; Liang, S.; Hu, L.; Guo, X.; Nie, Y.; Cao, J. Chromatin reorganization and endogenous auxin/cytokinin dynamic activity during somatic embryogenesis of cultured cotton cell. Plant Cell Tissue Organ Cult. 2007, 90, 63–70. [Google Scholar] [CrossRef]
- Reinert, J. Morphogenese und ihre Kontrolle an Gewebekulturen aus Carotten. Naturwissenschaften 1958, 45, 344–345. [Google Scholar] [CrossRef]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and Organized Development of Cultured Cells. II. Organization in Cultures Grown from Freely Suspended Cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Yuan, D.; Jin, F.; Zhang, Y.; Xu, J. Transcript profiling reveals complex auxin signalling pathway and transcription regulation involved in dedifferentiation and redifferentiation during somatic embryogenesis in cotton. BMC Plant Biol. 2012, 12, 110. [Google Scholar] [CrossRef]
- Indoliya, Y.; Tiwari, P.; Chauhan, A.S.; Goel, R.; Shri, M.; Bag, S.K.; Chakrabarty, D. Decoding regulatory landscape of somatic embryogenesis reveals differential regulatory networks between japonica and indica rice subspecies. Sci. Rep. 2016, 6, 23050. [Google Scholar] [CrossRef]
- Gaj, M.D.; Zhang, S.; Harada, J.J.; Lemaux, P.G. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 2005, 222, 977–988. [Google Scholar] [CrossRef]
- Lelu-Walter, M.-A.; Thompson, D.; Harvengt, L.; Sanchez, L.; Toribio, M.; Pâques, L.E. Somatic embryogenesis in forestry with a focus on Europe: State-of-the-art, benefits, challenges and future direction. Tree Genet. Genomes 2013, 9, 883–899. [Google Scholar] [CrossRef]
- Etienne, H. Somatic embryogenesis protocol: Coffee (Coffea arabica L. and C. canephora P.). In Protocols for Somatic Embryogenesis in Woody Plants; Jain, S.M., Gupta, P.K., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 167–179. [Google Scholar]
- Maximova, S.N.; Alemanno, L.; Young, A.; Ferriere, N.; Traore, A.; Guiltinan, M.J. Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis of Theobroma cacao L. In Vitro Cell. Dev. Biol. Plant 2002, 38, 252–259. [Google Scholar] [CrossRef]
- Montalbán, I.A.; De Diego, N.; Moncaleán, P. Enhancing initiation and proliferation in radiata pine (Pinus radiata D. Don) somatic embryogenesis through seed family screening, zygotic embryo staging and media adjustments. Acta Physiol. Plant. 2012, 34, 451–460. [Google Scholar] [CrossRef]
- Corredoira, E.; Ballester, A.; Ibarra, M.; Vieitez, A.M. Induction of somatic embryogenesis in explants of shoot cultures established from adult Eucalyptus globulus and E. saligna × E. maidenii trees. Tree Physiol. 2015, 35, 678–690. [Google Scholar] [CrossRef]
- Corredoira, E.; Valladares, S.; Vieitez, A.M. Morphohistological analysis of the origin and development of somatic embryos from leaves of mature Quercus robur. In Vitro Cell. Dev. Biol. Plant 2006, 42, 525–533. [Google Scholar] [CrossRef]
- Ribas, A.F.; Dechamp, E.; Champion, A.; Bertrand, B.; Combes, M.-C.; Verdeil, J.-L.; Lapeyre, F.; Lashermes, P.; Etienne, H. Agrobacterium-mediated genetic transformation of Coffea arabica (L.) is greatly enhanced by using established embryogenic callus cultures. BMC Plant Biol. 2011, 11, 92. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef]
- Boutilier, K.; Angenent, G.C.; Castan, M.S.; Hui, L. Haploid Embryogenesis. Patent US20160212956A1, 28 July 2016. [Google Scholar]
- Yang, X.; Zhang, X. Regulation of Somatic Embryogenesis in Higher Plants. Crit. Rev. Plant Sci. 2010, 29, 36–57. [Google Scholar] [CrossRef]
- Jayasankar, S.; Bondada, B.R.; Li, Z.; Gray, D.J. Comparative Anatomy and Morphology of Vitis vinifera (Vitaceae) Somatic Embryos from Solid- and Liquid-Culture-Derived Proembryogenic Masses. Am. J. Bot. 2003, 90, 973–979. [Google Scholar] [CrossRef]
- Raghavan, V. Role of 2,4-dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: Cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2,4-D. Am. J. Bot. 2004, 91, 1743–1756. [Google Scholar] [CrossRef]
- Florez, S.L.; Erwin, R.L.; Maximova, S.N.; Guiltinan, M.J.; Curtis, W.R. Enhanced somatic embryogenesis in Theobroma cacao using the homologous BABY BOOM transcription factor. BMC Plant Biol. 2015, 15, 121. [Google Scholar] [CrossRef]
- Campos, N.A.; Panis, B.; Carpentier, S.C. Somatic Embryogenesis in Coffee: The Evolution of Biotechnology and the Integration of Omics Technologies Offer Great Opportunities. Front. Plant Sci. 2017, 8, 1460. [Google Scholar] [CrossRef] [Green Version]
- Bonga, J.M.; Klimaszewska, K.K.; von Aderkas, P. Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Cult. 2010, 100, 241–254. [Google Scholar] [CrossRef]
- Etienne, H.; Breton, D.; Breitler, J.-C.; Bertrand, B.; Déchamp, E.; Awada, R.; Marraccini, P.; Léran, S.; Alpizar, E.; Campa, C.; et al. Coffee Somatic Embryogenesis: How Did Research, Experience Gained and Innovations Promote the Commercial Propagation of Elite Clones from the Two Cultivated Species? Front. Plant Sci. 2018, 9, 1630. [Google Scholar] [CrossRef]
- Aguilar-Hernández, V.; Loyola-Vargas, V.M. Advanced Proteomic Approaches to Elucidate Somatic Embryogenesis. Front. Plant Sci. 2018, 9, 1658. [Google Scholar] [CrossRef] [Green Version]
- Pais, M.S. Somatic Embryogenesis Induction in Woody Species: The Future After OMICs Data Assessment. Front. Plant Sci. 2019, 10, 240. [Google Scholar] [CrossRef] [Green Version]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar] [CrossRef]
- Businge, E.; Brackmann, K.; Moritz, T.; Egertsdotter, U. Metabolite profiling reveals clear metabolic changes during somatic embryo development of Norway spruce (Picea abies). Tree Physiol. 2012, 32, 232–244. [Google Scholar] [CrossRef] [Green Version]
- Robinson, A.R.; Dauwe, R.; Ukrainetz, N.K.; Cullis, I.F.; White, R.; Mansfield, S.D. Predicting the regenerative capacity of conifer somatic embryogenic cultures by metabolomics. Plant Biotechnol. J. 2009, 7, 952–963. [Google Scholar] [CrossRef]
- Dobrowolska, I.; Businge, E.; Abreu, I.N.; Moritz, T.; Egertsdotter, U. Metabolome and transcriptome profiling reveal new insights into somatic embryo germination in Norway spruce (Picea abies). Tree Physiol. 2017, 37, 1752–1766. [Google Scholar] [CrossRef]
- Vondrakova, Z.; Dobrev, P.I.; Pesek, B.; Fischerova, L.; Vagner, M.; Motyka, V. Profiles of Endogenous Phytohormones Over the Course of Norway Spruce Somatic Embryogenesis. Front. Plant Sci. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Gautier, F.; Label, P.; Eliášová, K.; Leplé, J.-C.; Motyka, V.; Boizot, N.; Vondráková, Z.; Malbeck, J.; Trávníčková, A.; Le Metté, C.; et al. Cytological, Biochemical and Molecular Events of the Embryogenic State in Douglas-fir (Pseudotsuga menziesii [Mirb.]). Front. Plant Sci. 2019, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, B.; Montagnon, C.; Georget, F.; Charmetant, P.; Etienne, H. Création et diffusion de variétés de caféiers Arabica: Quelles innovations variétales? Cah. Agric. 2012, 21, 77–88. [Google Scholar]
- Ducos, J.P.; Labbe, G.; Lambot, C.; Pétiard, V. Pilot scale process for the production of pre-germinated somatic embryos of selected robusta (Coffea canephora) clones. In Vitro Cell. Dev. Biol. Plant 2007, 43, 652–659. [Google Scholar] [CrossRef]
- Etienne, H.; Bertrand, B.; Montagnon, C.; Bodadilla Landey, R.; Dechamp, E.; Jourdan, I.; Alpizar, E.; Malo, E.; Georget, F. Un exemple de transfert de technologie réussi dans le domaine de la micropropagation: La multiplication de Coffea arabica par embryogenèse somatique. Cah. Agric. 2012, 21, 115–124. [Google Scholar]
- Ducos, J.P.; Alenton, R.; Reano, J.F.; Kanchanomai, C.; Deshayes, A.; Pétiard, V. Agronomic performance of Coffea canephora P. trees derived from large-scale somatic embryo production in liquid medium. Euphytica 2003, 131, 215–223. [Google Scholar] [CrossRef]
- Landey, R.B.; Cenci, A.; Georget, F.; Bertrand, B.; Camayo, G.; Dechamp, E.; Herrera, J.C.; Santoni, S.; Lashermes, P.; Simpson, J.; et al. High Genetic and Epigenetic Stability in Coffea arabica Plants Derived from Embryogenic Suspensions and Secondary Embryogenesis as Revealed by AFLP, MSAP and the Phenotypic Variation Rate. PLoS ONE 2013, 8, e56372. [Google Scholar]
- Georget, F.; Courtel, P.; Garcia, E.M.; Hidalgo, M.; Alpizar, E.; Breitler, J.-C.; Bertrand, B.; Etienne, H. Somatic embryogenesis-derived coffee plantlets can be efficiently propagated by horticultural rooted mini-cuttings: A boost for somatic embryogenesis. Sci. Hortic. 2017, 216, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Verdeil, J.-L.; Hocher, V.; Huet, C.; Grosdemange, F.; Escoute, J.; Ferrière, N.; Nicole, M. Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Ann. Bot. 2001, 88, 9–18. [Google Scholar] [CrossRef]
- Talamond, P.; Verdeil, J.-L.; Conéjéro, G. Secondary metabolite localization by autofluorescence in living plant cells. Molecules 2015, 20, 5024–5037. [Google Scholar] [CrossRef]
- Díaz-Sala, C. Molecular Dissection of the Regenerative Capacity of Forest Tree Species: Special Focus on Conifers. Front. Plant Sci. 2019, 9, 1943. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, K.; Xu, L.; Paszkowski, U.; Hayashi, M. Multifaceted Cellular Reprogramming at the Crossroads Between Plant Development and Biotic Interactions. Plant Cell Physiol. 2018, 59, 651–655. [Google Scholar] [CrossRef] [Green Version]
- Tao, L.; Zhao, Y.; Wu, Y.; Wang, Q.; Yuan, H.; Zhao, L.; Guo, W.; You, X. Transcriptome profiling and digital gene expression by deep sequencing in early somatic embryogenesis of endangered medicinal Eleutherococcus senticosus Maxim. Gene 2016, 578, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Trontin, J.-F.; Klimaszewska, K.; Morel, A.; Hargreaves, C.; Lelu-Walter, M.-A. Molecular Aspects of Conifer Zygotic and Somatic Embryo Development: A Review of Genome-Wide Approaches and Recent Insights. Methods Mol. Biol. 2016, 1359, 167–207. [Google Scholar] [PubMed]
- Nowak, K.; Wójcikowska, B.; Gaj, M.D. ERF022 impacts the induction of somatic embryogenesis in Arabidopsis through the ethylene-related pathway. Planta 2015, 241, 967–985. [Google Scholar] [CrossRef] [PubMed]
- Zavattieri, M.A.; Frederico, A.M.; Lima, M.; Sabino, R.; Arnholdt-Schmitt, B. Induction of somatic embryogenesis as an example of stress-related plant reactions. Electro. J. Biotech. 2010, 13, 12–13. [Google Scholar] [CrossRef]
- Nic-Can, G.I.; Galaz-Ávalos, R.M.; De-la-Peña, C.; Alcazar-Magaña, A.; Wrobel, K.; Loyola-Vargas, V.M. Somatic Embryogenesis: Identified Factors that Lead to Embryogenic Repression. A Case of Species of the Same Genus. PLoS ONE 2015, 10, e0126414. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garay, A.; Lopez, J.A.; Camafeita, E.; Bueno, M.A.; Pintos, B. Proteomic perspective of Quercus suber somatic embryogenesis. J. Proteom. 2013, 93, 314–325. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Silva, R.; Carmo, L.S.T.; Luis, Z.G.; Silva, L.P.; Scherwinski-Pereira, J.E.; Mehta, A. Proteomic identification of differentially expressed proteins during the acquisition of somatic embryogenesis in oil palm (Elaeis guineensis Jacq.). J. Proteom. 2014, 104, 112–127. [Google Scholar] [CrossRef]
- Xu, C.; Cao, H.; Xu, E.; Zhang, S.; Hu, Y. Genome-Wide Identification of Arabidopsis LBD29 Target Genes Reveals the Molecular Events behind Auxin-Induced Cell Reprogramming during Callus Formation. Plant Cell Physiol. 2018, 59, 744–755. [Google Scholar] [CrossRef]
- Gatica-Arias, A.M.; Arrieta-Espinoza, G.; Espinoza Esquivel, A.M. Plant regeneration via indirect somatic embryogenesis and optimisation of genetic transformation in Coffea arabica L. cvs. Caturra and Catuaí. Electron. J. Biotechnol. 2008, 11, 1–12. [Google Scholar] [CrossRef]
- Pádua, M.S.; Paiva, L.V.; Silva, L.C.D.; Livramento, K.G.D.; Alves, E.; Castro, A.H.F. Morphological characteristics and cell viability of coffee plants calli. Ciênc. Rural 2014, 44, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.T.; Barduche, D.; do Livramento, K.G.; Ligterink, W.; Paiva, L.V. Characterization of a Putative Serk-Like Ortholog in Embryogenic Cell Suspension Cultures of Coffea arabica L. Plant Mol. Biol. Rep. 2014, 32, 176–184. [Google Scholar] [CrossRef]
- Neves, N.; Segura-Nieto, M.; Blanco, M.A.; Sánchez, M.; González, A.; González, J.L.; Castillo, R. Biochemical characterization of embryogenic and non-embryogenic calluses of sugarcane. In Vitro Cell. Dev. Biol. Plant 2003, 39, 343–345. [Google Scholar] [CrossRef]
- Jeyaseelan, M.; Rao, M.V. Biochemical studies of embryogenic and non-embryogenic callus of Cardiospermum halicacabum L. Indian J. Exp. Biol. 2005, 43, 555–560. [Google Scholar]
- Vestman, D.; Larsson, E.; Uddenberg, D.; Cairney, J.; Clapham, D.; Sundberg, E.; von Arnold, S. Important processes during differentiation and early development of somatic embryos of Norway spruce as revealed by changes in global gene expression. BMC Proc. 2011, 5, P78. [Google Scholar] [CrossRef]
- Guillou, C.; Fillodeau, A.; Brulard, E.; Breton, D.; De Faria Maraschin, S.; Verdier, D.; Simon, M.; Ducos, J.-P. Indirect somatic embryogenesis of Theobroma cacao L. in liquid medium and improvement of embryo-to-plantlet conversion rate. In Vitro Cell. Dev. Biol. Plant 2018, 54, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline mechanisms of stress survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Magnani, E.; Jiménez-Gómez, J.M.; Soubigou-Taconnat, L.; Lepiniec, L.; Fiume, E. Profiling the onset of somatic embryogenesis in Arabidopsis. BMC Genom. 2017, 18, 998. [Google Scholar] [CrossRef]
- Von Aderkas, P.; Lelu, M.-A.; Label, P. Plant growth regulator levels during maturation of larch somatic embryos. Plant Physiol. Biochem. 2001, 39, 495–502. [Google Scholar] [CrossRef]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–Cytokinin Interaction Regulates Meristem Development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef]
- White, P.R. Potentially Unlimited Growth of Excised Plant Callus in an Artificial Nutrient. Am. J. Bot. 1939, 26, 59–64. [Google Scholar] [CrossRef]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 11, 118–130. [Google Scholar] [PubMed]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Guan, Y.; Li, S.-G.; Fan, X.-F.; Su, Z.-H. Application of Somatic Embryogenesis in Woody Plants. Front. Plant Sci. 2016, 7, 938. [Google Scholar] [CrossRef] [PubMed]
- Ladygin, V.G.; Bondarev, N.I.; Semenova, G.A.; Smolov, A.A.; Reshetnyak, O.V.; Nosov, A.M. Chloroplast ultrastructure, photosynthetic apparatus activities and production of steviol glycosides in Stevia rebaudiana in vivo and in vitro. Biol. Plant. 2008, 52, 9–16. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Hu, H.; Huang, X.; Zhang, Y.; Wang, Y.; Li, Z.; Zou, C.; Peng, H.; Li, L.; Gao, S.; et al. Metabolomic and Proteomic Analysis of Maize Embryonic Callus induced from immature embryo. Sci. Rep. 2017, 7, 1004. [Google Scholar] [CrossRef]
- Svobodová, H.; Albrechtová, J.; Kumstýřová, L.; Lipavská, H.; Vágner, M.; Vondráková, Z. Somatic embryogenesis in Norway spruce: Anatomical study of embryo development and influence of polyethylene glycol on maturation process. Plant Physiol. Bioch. 1999, 37, 209–221. [Google Scholar] [CrossRef]
- Fischerova, L.; Fischer, L.; Vondrakova, Z.; Vagner, M. Expression of the gene encoding transcription factor PaVP1 differs in Picea abies embryogenic lines depending on their ability to develop somatic embryos. Plant Cell Rep. 2008, 27, 435–441. [Google Scholar] [CrossRef]
- Iraqi, D.; Tremblay, F.M. Analysis of carbohydrate metabolism enzymes and cellular contents of sugars and proteins during spruce somatic embryogenesis suggests a regulatory role of exogenous sucrose in embryo development. J. Exp. Bot. 2001, 52, 2301–2311. [Google Scholar] [CrossRef] [Green Version]
- Dodeman, V.L.; Ducreux, G.; Kreis, M. Zygotic embryogenesis versus somatic embryogenesis. J. Exp. Bot. 1997, 48, 1493–1509. [Google Scholar]
- Pedroso, M.C.; Primikirios, N.; Roubelakis-Angelakis, K.A.; Pais, M.S. Free and conjugated polyamines in embryogenic and non-embryogenic leaf regions of camellia leaves before and during direct somatic embryogenesis. Physiol. Plant. 1997, 101, 213–219. [Google Scholar] [CrossRef]
- Grace, S.C.; Logan, B.A. Energy dissipation and radical scavenging by the plant phenylpropanoid pathway. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1499–1510. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Ikeda-Iwai, M.; Umehara, M.; Satoh, S.; Kamada, H. Stress-induced somatic embryogenesis in vegetative tissues of Arabidopsis thaliana. Plant J. 2003, 34, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Dethloff, F.; Erban, A.; Orf, I.; Alpers, J.; Fehrle, I.; Beine-Golovchuk, O.; Schmidt, S.; Schwachtje, J.; Kopka, J. Profiling methods to identify cold-regulated primary metabolites using gas chromatography coupled to mass spectrometry. Methods Mol. Biol. 2014, 1166, 171–197. [Google Scholar]
- Erban, A.; Schauer, N.; Fernie, A.R.; Kopka, J. Nonsupervised construction and application of mass spectral and retention time index libraries from time-of-flight gas chromatography-mass spectrometry metabolite profiles. Methods Mol. Biol. 2007, 358, 19–38. [Google Scholar] [PubMed]
- Luedemann, A.; Strassburg, K.; Erban, A.; Kopka, J. TagFinder for the quantitative analysis of gas chromatography—Mass spectrometry (GC-MS)-based metabolite profiling experiments. Bioinformatics 2008, 24, 732–737. [Google Scholar] [CrossRef]
- Kopka, J.; Schauer, N.; Krueger, S.; Birkemeyer, C.; Usadel, B.; Bergmüller, E.; Dörmann, P.; Weckwerth, W.; Gibon, Y.; Stitt, M.; et al. [email protected]: The Golm Metabolome Database. Bioinformatics 2005, 21, 1635–1638. [Google Scholar] [CrossRef]
- Strehmel, N.; Hummel, J.; Erban, A.; Strassburg, K.; Kopka, J. Retention index thresholds for compound matching in GC–MS metabolite profiling. J. Chromatogr. B 2008, 871, 182–190. [Google Scholar] [CrossRef]
- Campa, C.; Urban, L.; Mondolot, L.; Fabre, D.; Roques, S.; Lizzi, Y.; Aarrouf, J.; Doulbeau, S.; Breitler, J.-C.; Letrez, C.; et al. Juvenile Coffee Leaves Acclimated to Low Light Are Unable to Cope with a Moderate Light Increase. Front. Plant Sci. 2017, 8, 1126. [Google Scholar] [CrossRef]
- N.R.C. Canada. Plant Hormone Profiling. Available online: https://nrc.canada.ca/en/research-development/products-services/technical-advisory-services/plant-hormone-profiling (accessed on 20 June 2019).
- Lulsdorf, M.M.; Yuan, H.Y.; Slater, S.M.H.; Vandenberg, A.; Han, X.; Zaharia, L.I.; Abrams, S.R. Endogenous hormone profiles during early seed development of C. arietinum and C. anatolicum. Plant Growth Regul. 2013, 71, 191–198. [Google Scholar] [CrossRef]
- Chauvaux, N.; Van Dongen, W.; Esmans, E.L.; Van Onckelen, H.A. Quantitative analysis of 1-aminocyclopropane-1-carboxylic acid by liquid chromatography coupled to electrospray tandem mass spectrometry. J. Chromatogr. A 1997, 775, 143–150. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Cheadle, C.; Vawter, M.P.; Freed, W.J.; Becker, K.G. Analysis of Microarray Data Using Z Score Transformation. J. Mol. Diagn. 2003, 5, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CRAN—Package Pvclust. Available online: https://cran.r-project.org/web/packages/pvclust/index.html (accessed on 20 June 2019).
- Suzuki, R.; Shimodaira, H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 2006, 22, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- MetaboAnalyst. Available online: https://www.metaboanalyst.ca/MetaboAnalyst/faces/upload/Enrich UploadView.xhtml (accessed on 19 August 2019).

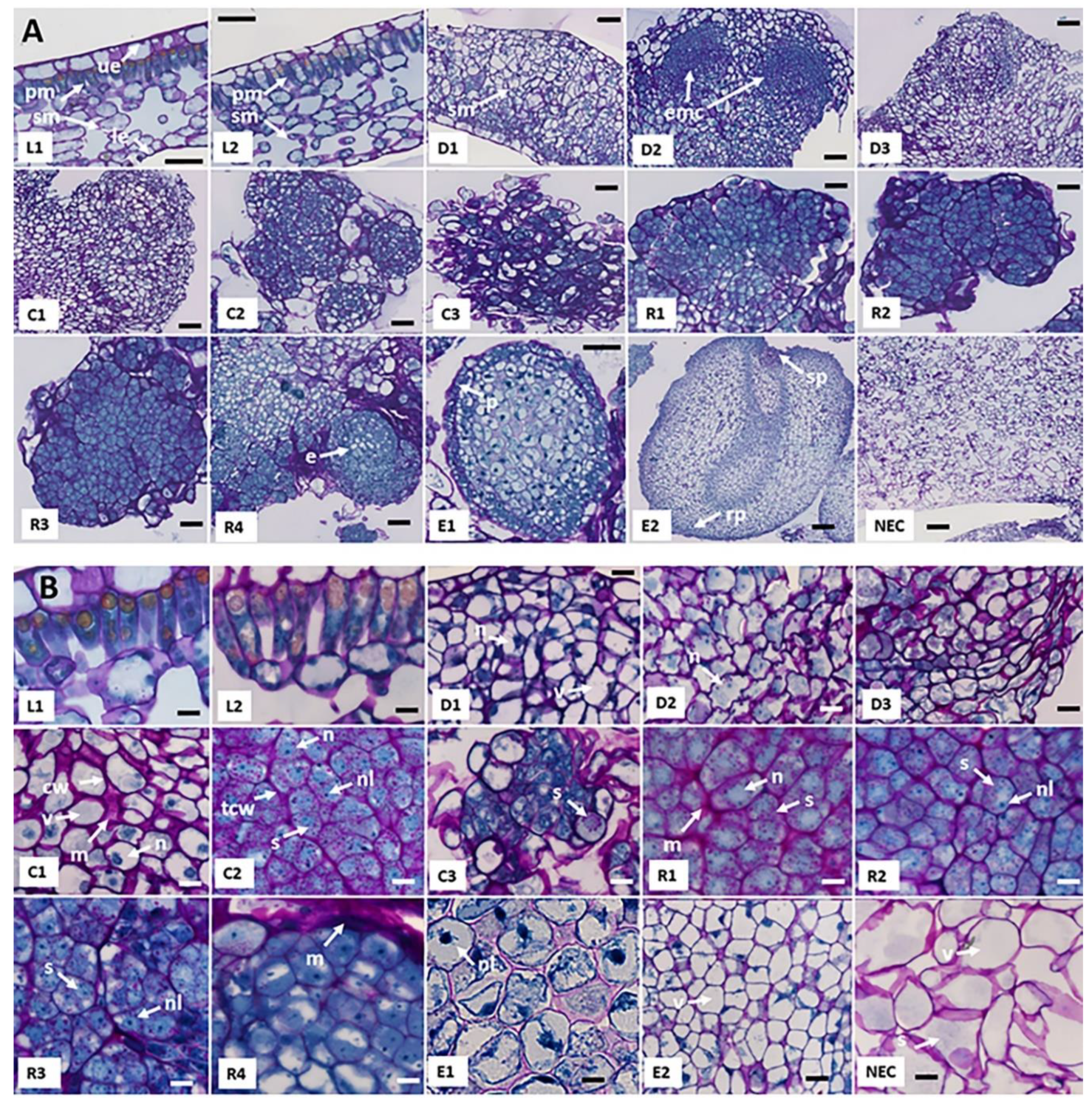

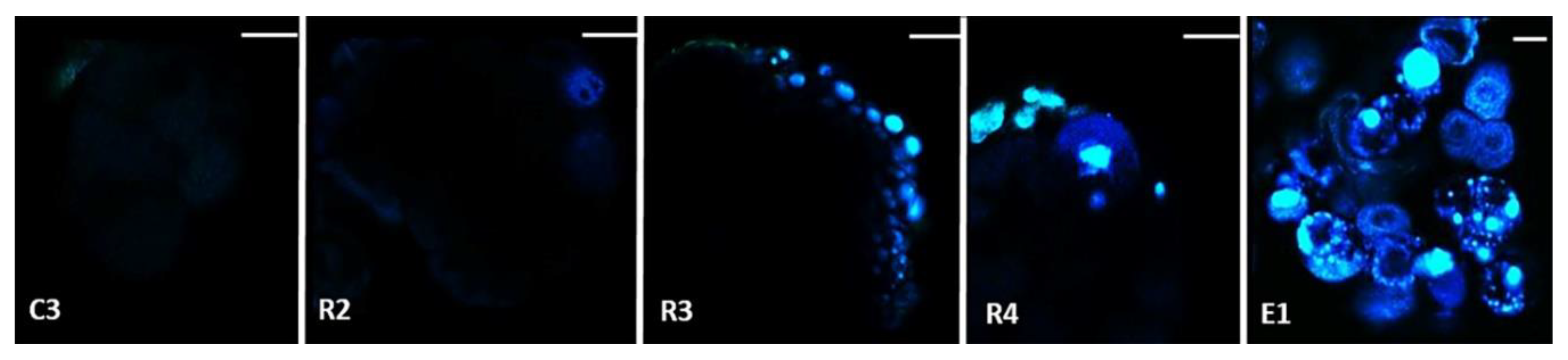
| Cell Type | Stage | Cell Length (µm) | Cell Width (µm) | W/L Ratio | Cytoplasm Size (µm2) | Nucleus Size (µm2) | N/C Ratio | Cell Division | Starch | Proteins |
|---|---|---|---|---|---|---|---|---|---|---|
| Palisade mesophyll cells | L1 | 41.5 ± 1.7 a | 13.9 ± 2.5 a | 0.33 ± 0.04 a | 430 ± 93 a | 66.5 ± 25 a | 0.17 ± 0.02 a | - | + | + |
| Palisade mesophyll cells | L2 | 38.2 ± 4.1 a | 13.0 ± 2.0 a | 0.35 ± 0.01 a | 440 ± 99 a | 59.1 ± 17 a | 0.19 ± 0.03 a | - | + | + |
| Dedifferentiated cells | D1 | 33.9 ± 2.5 a | 22.8 ± 4.0 b | 0.67 ± 0.08 b | 647 ± 71 b | 56.8 ± 7.4 a | 0.09 ± 0.02 a | +++ | + | + |
| Dedifferentiated cells | D2 | 32.7 ± 1.8 a | 22.4 ± 0.7 b | 0.69 ± 0.02 b | 681 ± 44 b | 155 ± 18.3 b | 0.24 ± 0.04 b | +++ | + | + |
| Dedifferentiated cells | D3 | 35.8 ± 2.6 a | 27.2 ± 5.1 b | 0.76 ± 0.11 b | 695 ± 91 b | 55.4 ± 11.3 a | 0.08 ± 0.03 a | +++ | ++ | + |
| Primary callus cells | C1 | 35.9 ± 2.1 a | 30.6 ± 2.6 b | 0.85 ± 0.02 b | 784 ± 56 b | 114 ± 25.3 b | 0.19 ± 0.03 a | + | ++ | + |
| Embryogenic cells | C2 | 20.2 ± 1.3 b | 14.7 ± 0.5 a | 0.73 ± 0.03 b | 214 ± 18 c | 70.7 ± 5.1 a | 0.33 ± 0.02 b | ++ | +++ | +++ |
| Embryogenic cell clusters | C3 | 23.9 ± 1.0 b | 12.7 ± 1.1 a | 0.54 ± 0.06 a | 223 ± 15 c | 70.1 ± 10.5 a | 0.31 ± 0.03 b | ++++ | +++ | +++ |
| Pro-embryogenic masses | R1 | 19.5 ± 2.7 b | 12.1 ± 0.5 a | 0.62 ± 0.04 b | 144 ± 15 c | 66.3 ± 8.4 a | 0.45 ± 0.07 b | +++ | +++ | +++ |
| Pro-embryogenic masses | R2 | 20.2 ± 1.8 b | 13.9 ± 1.5 a | 0.69 ± 0.54 b | 202 ± 23 c | 65.2 ± 10.8 a | 0.32 ± 0.02 b | +++ | +++ | +++ |
| Pro-embryogenic masses | R3 | 18.8 ± 1.3 b | 12.5 ± 0.3 a | 0.67 ± 0.03 b | 165 ± 13 c | 69.0 ± 15.7 a | 0.41 ± 0.07 b | +++ | +++ | +++ |
| Pro-embryogenic masses | R4 | 26.7 ± 1.4 b | 16.5 ± 1.6 a | 0.70 ± 0.07 b | 315 ± 26 c | 98.7 ± 6.6 a | 0.31 ± 0.03 b | +++ | +++ | +++ |
| Embryonic cells | E1 | 22.5 ± 1.0 b | 14.5 ± 1.6 a | 0.64 ± 0.04 b | 236 ± 24 c | 24.2 ± 4.2 c | 0.10 ± 0.01 a | +++ | + | +++ |
| Embryonic cells | E2 | 42.0 ± 1.5 a | 31.9 ± 0.7 b | 0.76 ± 0.04 b | 943 ± 55d | 88.5 ± 10.3 a | 0.09 ± 0.01 a | +++ | + | ++ |
| Metabolic Pathways | Developmental Phases of Coffee Somatic Embryogenesis | ||||
|---|---|---|---|---|---|
| Leaf | Dedifferentiation | Callus | Redifferentiation | Embryo | |
| TCA cycle | Aconitic acid | Aconitic acid | Fumaric acid | ||
| Citric acid | Malic acid | ||||
| Fumaric acid | Succinic acid | ||||
| Glutaric acid, 2-oxo- | |||||
| Malic acid | |||||
| Succinic acid | |||||
| Sugar metabolism | Glucose-6-phosphate | Glucose | Glucose-6-phosphate | Glucose-6-phosphate | |
| Mannose-6-phosphate | Fructose | Fructose-6-phosphate | Fructose-6-phosphate | ||
| Maltose | Maltotriose | Mannose-6-phosphate | Mannose-6-phosphate | ||
| Sucrose | Ribose | Ribose | Starch | ||
| Amino acid metabolism | Alanine, beta- | Alanine, beta- | Glycine | Leucine | Alanine |
| Aspartic acid | Aspartic acid | Methionine | Lysine | Glycine | |
| Asparagine | Proline | Proline | Leucine | ||
| Glycine | Lysine | ||||
| Glutamine | Methionine | ||||
| Valine | Proline | ||||
| Glutamate | |||||
| Fatty acid metabolism | Hexadecanoic acid | Glycerol | Glycerol-3-phosphate | Glycerol | |
| Octadecanoic acid | Glycerol-3-phosphate | ||||
| Glyceric acid | |||||
| Polyols | Inositol, myo- | Inositol, myo- | Inositol, myo- | ||
| Mannitol | |||||
| N-compounds | Putrescine | ||||
| Ascorbate and aldarate metabolism | Galactonic acid | Arabinonic acid | |||
| Threonic acid | Galactaric acid | ||||
| Galactonic acid | |||||
| Shikimate pathway | Quinic acid | Quinic acid | Tryptophan | Tryptophan | |
| Shikimic acid | Phenylalanine | Caffeic acid | |||
| Caffeic acid | Benzoic acid | ||||
| Alkaloid precursors | Nicotinic acid | Guanosine | |||
| Xanthosine | Xanthosine | ||||
| Inosine | |||||
| Alkaloids | Trigonelline | ||||
| Theobromine | |||||
| Caffeine | |||||
| Phenolic compounds | Mangiferin | 3-CQA | |||
| Catechin | 3,5-diCQA | ||||
| Epicatechin | 4-CQA | ||||
| 3-CQA | 4,5-diCQA | ||||
| 3,4-diCQA | 5-CQA | ||||
| 4-CQA | FQA | ||||
| FQA | |||||
| Metabolic Pathways | Developmental Phases of Coffee Somatic Embryogenesis | ||||
|---|---|---|---|---|---|
| Leaf | Dedifferentiation | Callus | Redifferentiation | Embryo | |
| TCA cycle | Fumaric acid | ||||
| Glutaric acid, 2-oxo- | |||||
| Malic acid | |||||
| Sugar metabolism | Fructose-6-phosphate | Glucose | Fructose-6-phosphate | Fructose | |
| Glucose-6-phosphate | Fructose | Glucose-6-phosphate | |||
| Mannose-6-phosphate | Starch | Mannose-6-phosphate | |||
| Fructose | Sucrose | ||||
| Ribose | |||||
| Amino acid metabolism | Leucine | Lysine | Leucine | Valine | |
| Lysine | Lysine | ||||
| Valine | |||||
| Ascorbate and aldarate metabolism | Arabinonic acid | ||||
| Galactonic acid | |||||
| Threonic acid | |||||
| Polyols | Inositol, myo- | ||||
| Hormones | Developmental Phases of Coffee Somatic Embryogenesis | ||||
|---|---|---|---|---|---|
| Leaf > | Dedifferentiation > | Callus > | Redifferentiation > | Embryo | |
| ABA | 583 ± 72 a | 113 ± 16 b | 74 ± 12 b | 70 ± 12 b | 600 ± 55 a |
| ABAGE | 243 ± 45 a | 121 ± 36 a | 675 ± 41 b | 671 ± 77 b | 1230 ± 103 c |
| ACC | 1281 ± 173 a | 19491 ± 447 b | 3063 ± 272 a | 3179 ± 193 a | 3065 ± 169 a |
| IAA | 34 ± 6 a | 182 ± 27 b | 17 ± 7 a | 60 ± 8 c | 81 ± 19 c |
| IBA | N.D. a | 4345 ± 385 b | N.D. a | N.D. a | N.D. a |
| iP | N.D. a | 4557 ± 441 b | N.D. a | N.D. a | N.D. a |
| Z | 2 ± 1 a | 17 ± 3 b | 2 ± 1 a | N.D. a | 4 ± 2 b |
| ZR | 6 ± 3 a | 13 ± 3 a | 22 ± 4 b | 15 ± 5 a | 60 ± 6 c |
| Cell Type | Stage | Cell Length (µm) | Cell Width (µm) | W/L Ratio | Cytoplasm Size (µm2) | Nucleus Size (µm2) | N/C Ratio | Cell Division | Starch | Proteins |
|---|---|---|---|---|---|---|---|---|---|---|
| Embryogenic cells | EC | 20.2 ± 1.3 a | 14.7 ± 0.5 a | 0.73 ± 0.03 a | 214 ± 18.0 a | 70.7 ± 5.1 a | 0.33 ± 0.02 a | ++ | ++ | +++ |
| Non-embryogenic cells | NEC | 69.7 ± 10.7 b | 41.3 ± 1.6 b | 0.60 ± 0.08 a | 2081 ± 386 b | 364 ± 53 b | 0.17 ± 0.02 b | +++ | +++ | + |
| Metabolic Pathways | Over-Accumulated Metabolites | Under-Accumulated Metabolites | ||
|---|---|---|---|---|
| Embryogenic Callus | Non-Embryogenic Callus | Embryogenic Callus | Non-Embryogenic Callus | |
| TCA cycle | Citric acid | Fumaric acid | ||
| Glutaric acid, 2-oxo- | Glutaric acid, 2-oxo- | |||
| Malic acid | Malic acid | |||
| Succinic acid | ||||
| Sugar metabolism | Glucose | Starch | Fructose-6-phosphate | Fructose-6-phosphate |
| Fructose | Ribose | Glucose-6-phosphate | Glucose-6-phosphate | |
| Maltotriose | Mannose-6-phosphate | Mannose-6-phosphate | ||
| Ribose | ||||
| Amino acid metabolism | Glycine | Alanine, beta- | Leucine | Proline |
| Methionine | Glycine | Valine | Valine | |
| Proline | Leucine | |||
| Methionine | ||||
| Fatty acid metabolism | Glycerol | Glycerol | ||
| Polyols | Inositol, myo- | Inositol, myo- | ||
| Ascorbate and aldarate metabolism | Arabinonic acid | Arabinonic acid | ||
| Threonic acid | Threonic acid | |||
| Galactonic acid | ||||
| Hormones | Embryogenic Callus | Non-Embryogenic Callus |
|---|---|---|
| ABA | 130 ± 14 a | 8 ± 2 b |
| ABAGE | 1200 ± 84 a | 24 ± 11 b |
| ACC | 3074 ± 96 a | 3016 ± 161 a |
| IAA | 25 ± 6 a | 29 ± 3 a |
| IBA | N.D. a | N.D. a |
| iP | N.D. a | N.D. a |
| Z | 2 ± 1 a | N.D. b |
| ZR | 40 ± 5 a | 10 ± 3 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awada, R.; Campa, C.; Gibault, E.; Déchamp, E.; Georget, F.; Lepelley, M.; Abdallah, C.; Erban, A.; Martinez-Seidel, F.; Kopka, J.; et al. Unravelling the Metabolic and Hormonal Machinery During Key Steps of Somatic Embryogenesis: A Case Study in Coffee. Int. J. Mol. Sci. 2019, 20, 4665. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194665
Awada R, Campa C, Gibault E, Déchamp E, Georget F, Lepelley M, Abdallah C, Erban A, Martinez-Seidel F, Kopka J, et al. Unravelling the Metabolic and Hormonal Machinery During Key Steps of Somatic Embryogenesis: A Case Study in Coffee. International Journal of Molecular Sciences. 2019; 20(19):4665. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194665
Chicago/Turabian StyleAwada, Rayan, Claudine Campa, Estelle Gibault, Eveline Déchamp, Frédéric Georget, Maud Lepelley, Cécile Abdallah, Alexander Erban, Federico Martinez-Seidel, Joachim Kopka, and et al. 2019. "Unravelling the Metabolic and Hormonal Machinery During Key Steps of Somatic Embryogenesis: A Case Study in Coffee" International Journal of Molecular Sciences 20, no. 19: 4665. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194665





