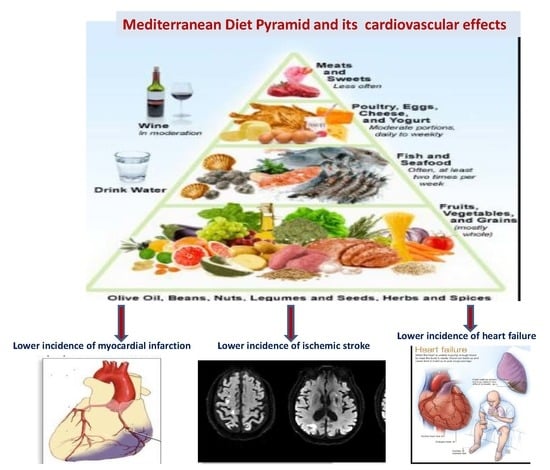
Metabolic and Vascular Effect of the Mediterranean Diet
Abstract
:1. Introduction: The Rediscovery of Mediterranean Diet
2. Biological Effects of Mediterranean Diet
2.1. Role of Genome
2.2. Role of Epigenome
2.3. Role of Nutrigenomics
2.4. Role of Microbiota
2.5. Effects on Inflammation Markers
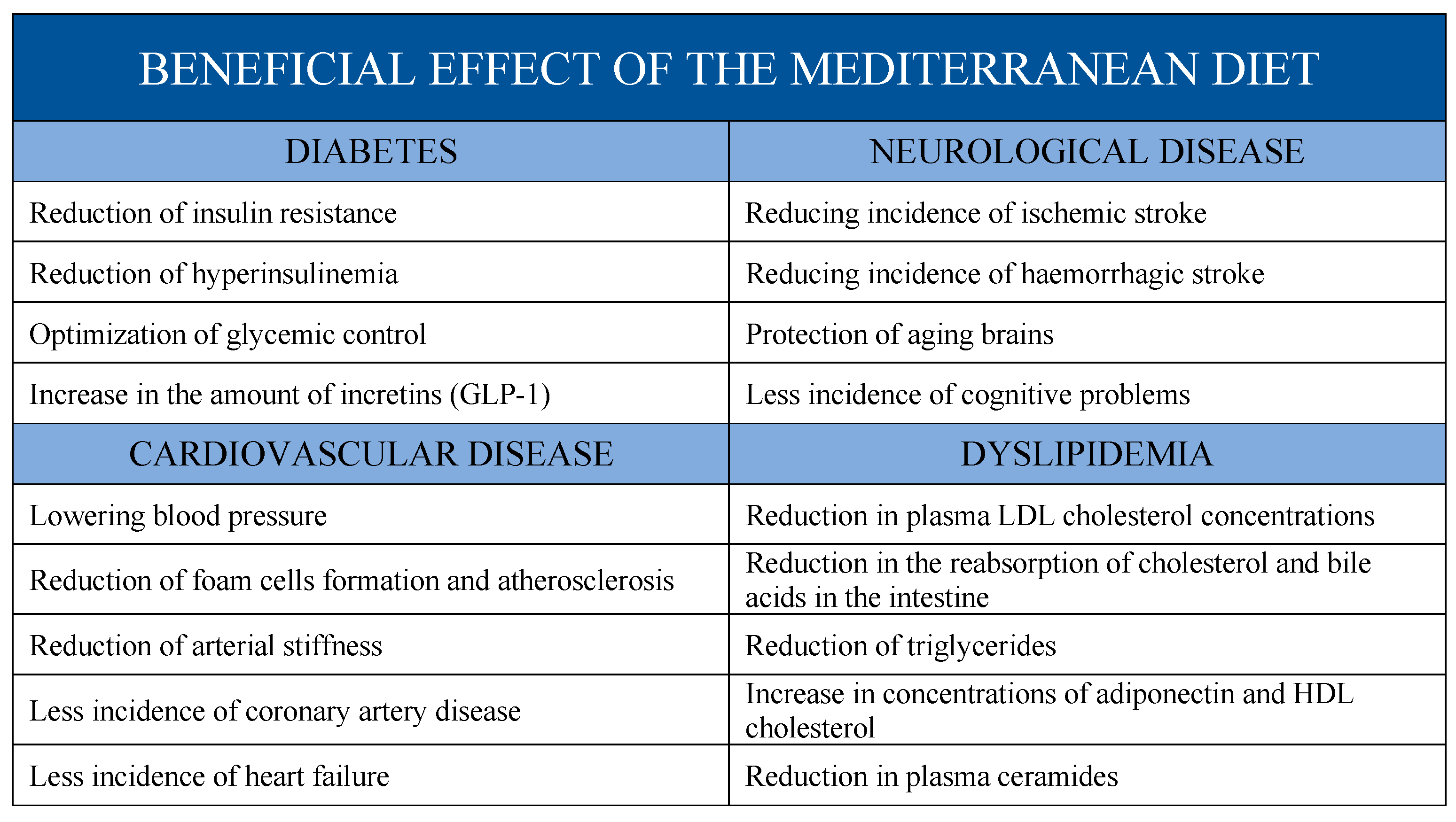
3. Effects of Mediterranean Diet on Cardiovascular Risk Factors
3.1. Mediterranean Diet and Diabetes
3.2. Mediterranean Diet and Hypertension
3.3. Mediterranean Diet and Lipid Levels
Effects on Plasma Ceramides
3.4. Effects of Mediterranean on Atherosclerosis
3.4.1. Role of Oxidative Stress
3.4.2. Effects of Mediterranean Diet on Foam Cells Formation
3.5. Mediterranean Diet and Arterial Stiffness
4. Mediterranean Diet and Coronary Artery Disease
5. Mediterranean Diet and Congestive Heart Failure
6. Mediterranean Diet and Stroke
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Keys, A. Coronary heart disease in seven countries. 1970. Nutr. Burbank Los Angel. Cty. Calif 1997, 13, 250–252. [Google Scholar]
- Buzina, R.; Keys, A.; Mohacek, I.; Marinkovic, M.; Hahn, A.; Blackburn, H. Coronary Heart Disease in Seven Countries. V. Five-year follow-up in Dalmatia and Slavonia. Available online: https://eurekamag.com/research/042/685/042685152.php (accessed on 3 September 2019).
- Fitó, M.; Konstantinidou, V. Nutritional Genomics and the Mediterranean Diet’s Effects on Human Cardiovascular Health. Nutrients 2016, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Keys, A. Mediterranean diet and public health: Personal reflections. Am. J. Clin. Nutr. 1995, 61, 1321S–1323S. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Aravanis, C.; Blackburn, H.W.; Van Buchem, F.S.; Buzina, R.; Djordjević, B.D.; Dontas, A.S.; Fidanza, F.; Karvonen, M.J.; Kimura, N.; et al. Epidemiological studies related to coronary heart disease: Characteristics of men aged 40–59 in seven countries. Acta Med. Scand. 1966, 460, 1–392. [Google Scholar] [CrossRef]
- Menotti, A.; Keys, A.; Aravanis, C.; Blackburn, H.; Dontas, A.; Fidanza, F.; Karvonen, M.J.; Kromhout, D.; Nedeljkovic, S.; Nissinen, A. Seven Countries Study. First 20-year mortality data in 12 cohorts of six countries. Ann. Med. 1989, 21, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Moorthy, M.V.; Demler, O.V.; Hu, F.B.; Ridker, P.M.; Chasman, D.I.; Mora, S. Assessment of Risk Factors and Biomarkers Associated with Risk of Cardiovascular Disease Among Women Consuming a Mediterranean Diet. JAMA Netw. Open 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, M.E.; Dedoussis, G.V.Z. Gene–Diet Interactions in Cardiovascular Disease. Curr. Nutr. Rep. 2012, 1, 153–160. [Google Scholar] [CrossRef]
- Linseisen, J.; Kesse, E.; Slimani, N.; Bueno-De-Mesquita, H.B.; Ocké, M.C.; Skeie, G.; Kumle, M.; Dorronsoro Iraeta, M.; Morote Gómez, P.; Janzon, L.; et al. Meat consumption in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts: Results from 24-h dietary recalls. Public Health Nutr. 2002, 5, 1243–1258. [Google Scholar] [CrossRef]
- La dieta mediterranea. Come mangiare bene e stare bene - Ancel Keys - Margaret Keys - Libro - Slow Food - AsSaggi IBS. Available online: https://www.ibs.it/dieta-mediterranea-come-mangiare-bene-libro-ancel-keys-margaret-keys/e/9788884994738 (accessed on 3 September 2019).
- Pereira-da-Silva, L.; Pinto, E. Low Adherence to Mediterranean Diet in Portugal: Pregnant Women Nutrition in Portugal and its Repercussions. Acta Med. Port. 2016, 29, 658–666. [Google Scholar] [CrossRef] [Green Version]
- Benhammou, S.; Monteagudo, C.; Mariscal-Arcas, M.; Ortega, V.; Rivas, A.; Ortega, E.; Lorenzo, M.L.; Olea-Serrano, F. Seguimiento de la dieta mediterránea e hidratación de la población española y marroquí. Nutr. Hosp. 2015, 32, 2749–2756. [Google Scholar]
- Balanza, R.; García-Lorda, P.; Pérez-Rodrigo, C.; Aranceta, J.; Bonet, M.B.; Salas-Salvadó, J. Trends in food availability determined by the Food and Agriculture Organization’s food balance sheets in Mediterranean Europe in comparison with other European areas. Public Health Nutr. 2007, 10, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Belahsen, R.; Rguibi, M. Population health and Mediterranean diet in southern Mediterranean countries. Public Health Nutr. 2006, 9, 1130–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhammou, S.; Heras-González, L.; Ibáñez-Peinado, D.; Barceló, C.; Hamdan, M.; Rivas, A.; Mariscal-Arcas, M.; Olea-Serrano, F.; Monteagudo, C. Comparison of Mediterranean diet compliance between European and non-European populations in the Mediterranean basin. Appetite 2016, 107, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Dedoussis, G.V.; Kanoni, S.; Mariani, E.; Cattini, L.; Herbein, G.; Fulop, T.; Varin, A.; Rink, L.; Jajte, J.; Monti, D.; et al. Mediterranean diet and plasma concentration of inflammatory markers in old and very old subjects in the ZINCAGE population study. Clin. Chem. Lab. Med. 2008, 46, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, S.; Scholten, A.M.; Korobili, C.; Kyrli, D.; Tsigga, M.; Van Dieijen, T.; Kotzamanidis, C.; Grammatikopoulou, M.G. Greater Mediterranean diet adherence is observed in Dutch compared with Greek university students. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Kafatos, A.; Diacatou, A.; Voukiklaris, G.; Nikolakakis, N.; Vlachonikolis, J.; Kounali, D.; Mamalakis, G.; Dontas, A.S. Heart disease risk-factor status and dietary changes in the Cretan population over the past 30 y: The Seven Countries Study. Am. J. Clin. Nutr. 1997, 65, 1882–1886. [Google Scholar] [CrossRef]
- Physical activity and health in Europe: Evidence for action. Available online: http://www.euro.who.int/en/publications/abstracts/physical-activity-and-health-in-europe-evidence-for-action (accessed on 3 September 2019).
- Kyriacou, A.; Evans, J.M.M.; Economides, N.; Kyriacou, A. Adherence to the Mediterranean diet by the Greek and Cypriot population: A systematic review. Eur. J. Public Health 2015, 25, 1012–1018. [Google Scholar] [CrossRef]
- Sila, S.; Pavić, A.M.; Hojsak, I.; Ilić, A.; Pavić, I.; Kolaček, S. Comparison of Obesity Prevalence and Dietary Intake in School-Aged Children Living in Rural and Urban Area of Croatia. Prev. Nutr. Food Sci. 2018, 23, 282–287. [Google Scholar]
- Cambien, F.; Poirier, O.; Lecerf, L.; Evans, A.; Cambou, J.P.; Arveiler, D.; Luc, G.; Bard, J.M.; Bara, L.; Ricard, S. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature 1992, 359, 641–644. [Google Scholar] [CrossRef]
- Song, Y.; Stampfer, M.J.; Liu, S. Meta-analysis: Apolipoprotein E genotypes and risk for coronary heart disease. Ann. Intern. Med. 2004, 141, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Manolio, T.A. How to interpret a genome-wide association study. JAMA 2008, 299, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Carrasco, P.; Sorlí, J.V.; Estruch, R.; Rico-Sanz, J.; MARTinez-GONZalez, M.A.; Salas-Salvadó, J.; Covas, M.I.; Coltell, O.; Arós, F.; et al. Mediterranean diet reduces the adverse effect of the TCF7L2-rs7903146 polymorphism on cardiovascular risk factors and stroke incidence: A randomize. Diabetes Care 2013, 36, 3803–3811. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pubmed/23942586 (accessed on 3 September 2019). [CrossRef]
- Ortega-Azorín, C.; Sorlí, J.V.; Estruch, R.; Asensio, E.M.; Coltell, O.; González, J.I.; Martínez-González, M.Á.; Ros, E.; Salas-Salvadó, J.; Fitó, M.; et al. Amino acid change in the carbohydrate response element binding protein is associated with lower triglycerides and myocardial infarction incidence depending on level of adherence to the Mediterranean diet in the PREDIMED trial. Circ. Cardiovasc. Genet. 2014, 7, 49–58. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Martínez-González, M.A.; Razquin, C.; Arós, F.; Lapetra, J.; Martínez, J.A.; Zalba, G.; Marti, A. Mediterranean diet and telomere length in high cardiovascular risk subjects from the PREDIMED-NAVARRA study. Clin. Nutr. Edinb. Scotl. 2016, 35, 1399–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccardi, V.; Esposito, A.; Rizzo, M.R.; Marfella, R.; Barbieri, M.; Paolisso, G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS ONE 2013, 8, e62781. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; De Vivo, I. Mediterranean diet and telomere length in Nurses’ Health Study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Delgado-Lista, J.; Ramirez, R.; Carracedo, J.; Caballero, J.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Garcia-Rios, A.; Delgado-Casado, N.; Cruz-Teno, C.; et al. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age Dordr. Neth. 2012, 34, 1309–1316. [Google Scholar] [CrossRef]
- García-Calzón, S.; Martínez-González, M.A.; Razquin, C.; Corella, D.; Salas-Salvadó, J.; Martínez, J.A.; Zalba, G.; Marti, A. Pro12Ala polymorphism of the PPARγ2 gene interacts with a mediterranean diet to prevent telomere shortening in the PREDIMED-NAVARRA randomized trial. Circ. Cardiovasc. Genet. 2015, 8, 91–99. [Google Scholar] [CrossRef]
- Garcia-Rios, A.; Gomez-Delgado, F.J.; Garaulet, M.; Alcala-Diaz, J.F.; Delgado-Lista, F.J.; Marin, C.; Rangel-Zuñiga, O.A.; Rodriguez-Cantalejo, F.; Gomez-Luna, P.; Ordovas, J.M.; et al. Beneficial effect of CLOCK gene polymorphism rs1801260 in combination with low-fat diet on insulin metabolism in the patients with metabolic syndrome. Chronobiol. Int. 2014, 31, 401–408. [Google Scholar] [CrossRef]
- López-Guimerà, G.; Dashti, H.S.; Smith, C.E.; Sánchez-Carracedo, D.; Ordovas, J.M.; Garaulet, M. CLOCK 3111 T/C SNP interacts with emotional eating behavior for weight-loss in a Mediterranean population. PLoS ONE 2014, 9, e99152. [Google Scholar] [CrossRef]
- Jackson, A.A.; Burdge, G.C.; Lillycrop, K.A. Diet, Nutrition and Modulation of Genomic Expression in Fetal Origins of Adult Disease. J. Nutr. Nutr. 2011, 3, 192–208. [Google Scholar]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkemade, F.E.; van Vliet, P.; Henneman, P.; van Dijk, K.W.; Hierck, B.P.; van Munsteren, J.C.; Scheerman, J.A.; Goeman, J.J.; Havekes, L.M.; Gittenberger-de Groot, A.C.; et al. Prenatal exposure to apoE deficiency and postnatal hypercholesterolemia are associated with altered cell-specific lysine methyltransferase and histone methylation patterns in the vasculature. Am. J. Pathol. 2010, 176, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Lund, G.; Andersson, L.; Lauria, M.; Lindholm, M.; Fraga, M.F.; Villar-Garea, A.; Ballestar, E.; Esteller, M.; Zaina, S. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J. Biol. Chem. 2004, 279, 29147–29154. [Google Scholar] [CrossRef] [PubMed]
- Roadmap, E.; Consortium, K.A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; et al. Integrative analysis of 111 reference human epigenomes. Nature 2015, 518, 317–330. [Google Scholar] [Green Version]
- Lillycrop, K.A.; Hoile, S.P.; Grenfell, L.; Burdge, G.C. DNA methylation, ageing and the influence of early life nutrition. Proc. Nutr. Soc. 2014, 73, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Gallach, S.; Calabuig-Fariñas, S.; Jantus-Lewintre, E.; Camps, C. MicroRNAs: Promising new antiangiogenic targets in cancer. BioMed Res. Int. 2014, 2014, 878450. [Google Scholar] [CrossRef]
- Corella, D.; Ordovás, J.M. How does the Mediterranean diet promote cardiovascular health? Current progress toward molecular mechanisms: Gene-diet interactions at the genomic, transcriptomic, and epigenomic levels provide novel insights into new mechanisms. BioEssays News Rev. Mol. Cell. Dev. Biol. 2014, 36, 526–537. [Google Scholar] [CrossRef]
- Bünger, M.; Hooiveld, G.J.E.J.; Kersten, S.; Müller, M. Exploration of PPAR functions by microarray technology--a paradigm for nutrigenomics. Biochim. Biophys. Acta 2007, 1771, 1046–1064. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.-I.; Sola, R.; Fitó, M. Up-to date knowledge on the in vivo transcriptomic effect of the Mediterranean diet in humans. Mol. Nutr. Food Res. 2013, 57, 772–783. [Google Scholar] [CrossRef]
- Garcia-Aloy, M.; Llorach, R.; Urpi-Sarda, M.; Jáuregui, O.; Corella, D.; Ruiz-Canela, M.; Salas-Salvadó, J.; Fitó, M.; Ros, E.; Estruch, R.; et al. A metabolomics-driven approach to predict cocoa product consumption by designing a multimetabolite biomarker model in free-living subjects from the PREDIMED study. Mol. Nutr. Food Res. 2015, 59, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Zuker, C.S. Food for the brain. Cell 2015, 161, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Legarrea, P.; Fuller, N.R.; Zulet, M.A.; Martinez, J.A.; Caterson, I.D. The influence of Mediterranean, carbohydrate and high protein diets on gut microbiota composition in the treatment of obesity and associated inflammatory state. Asia Pac. J. Clin. Nutr. 2014, 23, 360–368. [Google Scholar] [PubMed]
- Jin, Q.; Black, A.; Kales, S.N.; Vattem, D.; Ruiz-Canela, M.; Sotos-Prieto, M. Metabolomics and Microbiomes as Potential Tools to Evaluate the Effects of the Mediterranean Diet. Nutrients 2019, 11, 207. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pubmed/30669673 (accessed on 3 September 2019). [CrossRef] [PubMed]
- González-Guardia, L.; Yubero-Serrano, E.M.; Delgado-Lista, J.; Perez-Martinez, P.; Garcia-Rios, A.; Marin, C.; Camargo, A.; Delgado-Casado, N.; Roche, H.M.; Perez-Jimenez, F.; et al. Effects of the Mediterranean diet supplemented with coenzyme q10 on metabolomic profiles in elderly men and women. J. Gerontol. A. Biol. Sci. Med. Sci. 2015, 70, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Kakkoura, M.G.; Sokratous, K.; Demetriou, C.A.; Loizidou, M.A.; Loucaides, G.; Kakouri, E.; Hadjisavvas, A.; Kyriacou, K. Mediterranean diet-gene interactions: A targeted metabolomics study in Greek-Cypriot women. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Aguilera, E.; Urpi-Sarda, M.; Llorach, R.; Vázquez-Fresno, R.; Garcia-Aloy, M.; Carmona, F.; Sanchez, A.; Madrid-Gambin, F.; Estruch, R.; Corella, D.; et al. Microbial metabolites are associated with a high adherence to a Mediterranean dietary pattern using a 1H-NMR-based untargeted metabolomics approach. J. Nutr. Biochem. 2017, 48, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferré, M.; Zheng, Y.; Ruiz-Canela, M.; Hruby, A.; Martínez-González, M.A.; Clish, C.B.; Corella, D.; Estruch, R.; Ros, E.; Fitó, M.; et al. Plasma acylcarnitines and risk of cardiovascular disease: Effect of Mediterranean diet interventions. Am. J. Clin. Nutr. 2016, 103, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Hu, F.B.; Ruiz-Canela, M.; Bulló, M.; Toledo, E.; Wang, D.D.; Corella, D.; Gómez-Gracia, E.; Fiol, M.; Estruch, R.; et al. Plasma Metabolites from Choline Pathway and Risk of Cardiovascular Disease in the PREDIMED (Prevention with Mediterranean Diet) Study. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Wang, D.D.; Toledo, E.; Hruby, A.; Rosner, B.A.; Willett, W.C.; Sun, Q.; Razquin, C.; Zheng, Y.; Ruiz-Canela, M.; Guasch-Ferré, M.; et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevención con Dieta Mediterránea). Circulation 2017, 135, 2028–2040. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, F.B.; Ruiz-Canela, M.; Clish, C.B.; Dennis, C.; Salas-Salvado, J.; Hruby, A.; Liang, L.; Toledo, E.; Corella, D.; et al. Metabolites of Glutamate Metabolism Are Associated with Incident Cardiovascular Events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) Trial. J. Am. Heart Assoc. 2016, 5. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.L.; Yap, Y.A.; McLeod, K.H.; Mackay, C.R.; Mariño, E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef] [PubMed]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; García-Carpintero, S.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Landa, B.B.; Clemente, J.C.; Pérez-Martínez, P.; López-Miranda, J.; Pérez-Jiménez, F.; Camargo, A. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinction in the gut microbiota compounds over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Wagner, V.E.; Blanton, L.V.; Cheng, J.; Fontana, L.; Haque, R.; Ahmed, T.; Gordon, J.I. Regulators of gut motility revealed by a gnotobiotic model of diet-microbiome interactions related to travel. Cell 2015, 163, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Griffin, N.W.; Ahern, P.P.; Cheng, J.; Heath, A.C.; Ilkayeva, O.; Newgard, C.B.; Fontana, L.; Gordon, J.I. Prior Dietary Practices and Connections to a Human Gut Microbial Metacommunity Alter Responses to Diet Interventions. Cell Host Microbe 2017, 21, 84–96. [Google Scholar] [CrossRef]
- Esposito, K.; Marfella, R.; Ciotola, M.; Di Palo, C.; Giugliano, F.; Giugliano, G.; D’Armiento, M.; D’Andrea, F.; Giugliano, D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: A randomized trial. JAMA 2004, 292, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Blankenberg, S.; Barbaux, S.; Tiret, L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003, 170, 191–203. [Google Scholar] [CrossRef]
- Cortés, B.; Núñez, I.; Cofán, M.; Gilabert, R.; Pérez-Heras, A.; Casals, E.; Deulofeu, R.; Ros, E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J. Am. Coll. Cardiol. 2006, 48, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R. Anti-inflammatory effects of the Mediterranean diet: The experience of the PREDIMED study. Proc. Nutr. Soc. 2010, 69, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, M.A.; Siculella, L.; Ancora, M.A.; Massaro, M.; Scoditti, E.; Storelli, C.; Visioli, F.; Distante, A.; De Caterina, R. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation: Antiatherogenic properties of Mediterranean diet phytochemicals. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Núñez, I.; Pérez-Heras, A.; Serra, M.; Gilabert, R.; Casals, E.; Deulofeu, R. A walnut diet improves endothelial function in hypercholesterolemic subjects: A randomized crossover trial. Circulation 2004, 109, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Mena, M.-P.; Sacanella, E.; Vazquez-Agell, M.; Morales, M.; Fitó, M.; Escoda, R.; Serrano-Martínez, M.; Salas-Salvadó, J.; Benages, N.; Casas, R.; et al. Inhibition of circulating immune cell activation: A molecular antiinflammatory effect of the Mediterranean diet. Am. J. Clin. Nutr. 2009, 89, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Mitro, N.; Scurati, S.; Masciadri, M.; Mussoni, L.; Galli, G.V.; Bosisio, E.; Crestani, M.; De Fabiani, E.; et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J. Agric. Food Chem. 2006, 54, 3259–3264. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Elhayany, A.; Lustman, A.; Abel, R.; Attal-Singer, J.; Vinker, S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes. Metab. 2010, 12, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. The role of the gut microbiota in energy metabolism and metabolic disease. Curr. Pharm. Des. 2009, 15, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C. Dietary fatty acids and insulin resistance. Curr. Atheroscler. Rep. 1999, 1, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Brown-Borg, H.M.; Buffenstein, R. Cutting back on the essentials: Can manipulating intake of specific amino acids modulate health and lifespan? Ageing Res. Rev. 2017, 39, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Due, A.; Larsen, T.M.; Hermansen, K.; Stender, S.; Holst, J.J.; Toubro, S.; Martinussen, T.; Astrup, A. Comparison of the effects on insulin resistance and glucose tolerance of 6-mo high-monounsaturated-fat, low-fat, and control diets. Am. J. Clin. Nutr. 2008, 87, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, J.A.; de la Sacristana, A.G.; Sánchez, E.; Romero, I.; Vidal-Puig, A.; Berral, F.J.; Escribano, A.; Moyano, M.J.; Peréz-Martinez, P.; López-Miranda, J.; et al. A MUFA-rich diet improves posprandial glucose, lipid and GLP-1 responses in insulin-resistant subjects. J. Am. Coll. Nutr. 2007, 26, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Vessby, B.; Uusitupa, M.; Hermansen, K.; Riccardi, G.; Rivellese, A.A.; Tapsell, L.C.; Nälsén, C.; Berglund, L.; Louheranta, A.; Rasmussen, B.M.; et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: The KANWU Study. Diabetologia 2001, 44, 312–319. [Google Scholar] [CrossRef]
- Vessby, B. Dietary fat and insulin action in humans. Br. J. Nutr. 2000, 83 (Suppl. 1), S91–S96. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef]
- Christiansen, E.; Schnider, S.; Palmvig, B.; Tauber-Lassen, E.; Pedersen, O. Intake of a diet high in trans monounsaturated fatty acids or saturated fatty acids. Effects on postprandial insulinemia and glycemia in obese patients with NIDDM. Diabetes Care 1997, 20, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Garg, A. High-monounsaturated-fat diets for patients with diabetes mellitus: A meta-analysis. Am. J. Clin. Nutr. 1998, 67, 577S–582S. [Google Scholar] [CrossRef] [PubMed]
- Ros, E. Dietary cis-monounsaturated fatty acids and metabolic control in type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 617S–625S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehm, B.J.; Lattin, B.L.; Summer, S.S.; Boback, J.A.; Gilchrist, G.M.; Jandacek, R.J.; D’Alessio, D.A. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care 2009, 32, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Rasmussen, O.; Christiansen, C.; Pedersen, E.; Vesterlund, M.; Storm, H.; Ingerslev, J.; Hermansen, K. Comparison of the effects of a monounsaturated fat diet and a high carbohydrate diet on cardiovascular risk factors in first degree relatives to type-2 diabetic subjects. Eur. J. Clin. Nutr. 1999, 53, 818–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, M.; Adams-Huet, B.; Linda, B.; Scott, M.G.; Abhimanyu, G. Lipid, Glycemic, and Insulin Responses to Meals Rich in Saturated, Cis-Monounsaturated, and Polyunsaturated (n-3 and n-6) Fatty Acids in Subjects with Type 2 Diabetes. Diabetes Care 2007, 30, 2993–2998. [Google Scholar] [CrossRef]
- Pérez-Jiménez, F.; López-Miranda, M.D.; Pinillos, P.; Gómez, E.; Paz-Rojas, P.; Montilla, C.; Marín, M.J.; Velasco, A.; Blanco-Molina, J.A.; Jiménez, P.; et al. A Mediterranean and a High-Carbohydrate Diet Improve Glucose Metabolism in Healthy Young Persons. Diabetologia 2001, 44, 2038–2043. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041. [Google Scholar]
- Mancia, G.; Fagard, R.; Narkiewicz, K.; Redón, J.; Zanchetti, A.; Böhm, M.; Christiaens, T.; Cifkova, R.; De Backer, G.; Dominiczak, A.; et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 2013, 31, 1281–1357. [Google Scholar] [CrossRef]
- Dickinson, H.O.; Mason, J.M.; Nicolson, D.J.; Campbell, F.; Beyer, F.R.; Cook, J.V.; Williams, B.; Ford, G.A. Lifestyle interventions to reduce raised blood pressure: A systematic review of randomized controlled trials. J. Hypertens. 2006, 24, 215–233. [Google Scholar] [CrossRef]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Nissensohn, M.; Román-Viñas, B.; Sánchez-Villegas, A.; Piscopo, S.; Serra-Majem, L. The Effect of the Mediterranean Diet on Hypertension: A Systematic Review and Meta-Analysis. J. Nutr. Educ. Behav. 2016, 48, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Hu, F.B.; Estruch, R.; Buil-Cosiales, P.; Corella, D.; Salas-Salvadó, J.; Covas, M.I.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: Results from a randomized controlled trial. BMC Med. 2013, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Buga, G.M.; Wood, K.S.; Byrns, R.E.; Chaudhuri, G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. USA 1987, 84, 9265–9269. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 288, 373–376. [Google Scholar] [CrossRef]
- Brunner, H.; Cockcroft, J.R.; Deanfield, J.; Donald, A.; Ferrannini, E.; Halcox, J.; Kiowski, W.; Lüscher, T.F.; Mancia, G.; Natali, A.; et al. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 233–246. [Google Scholar] [CrossRef]
- Dhaun, N.; Goddard, J.; Kohan, D.E.; Pollock, D.M.; Schiffrin, E.L.; Webb, D.J. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension 2008, 52, 452–459. [Google Scholar] [CrossRef]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Fitó, M.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.-I.; Muñoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos, M.d.C.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflug. Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Delgado-Lista, J.; Garcia-Rios, A.; Cruz-Teno, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Lora-Aguilar, P.; Rodriguez-Cantalejo, F.; Fuentes-Jimenez, F.; et al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br. J. Nutr. 2012, 108, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Castañer, O.; Corella, D.; Covas, M.-I.; Sorlí, J.V.; Subirana, I.; Flores-Mateo, G.; Nonell, L.; Bulló, M.; de la Torre, R.; Portolés, O.; et al. In vivo transcriptomic profile after a Mediterranean diet in high-cardiovascular risk patients: A randomized controlled trial. Am. J. Clin. Nutr. 2013, 98, 845–853. [Google Scholar] [PubMed]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Ottlecz, A.; Lambrou, G.; Grueninger, M.; Finley, J.; Thompson, H.W.; Bazan, N.G. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4163–4170. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Luna, R.; Muñoz-Hernandez, R.; Miranda, M.L.; Costa, A.F.; Jimenez-Jimenez, L.; Vallejo-Vaz, A.J.; Muriana, F.J.G.; Villar, J.; Stiefel, P. Olive oil polyphenols decrease blood pressure and improve endothelial function in young women with mild hypertension. Am. J. Hypertens. 2012, 25, 1299–1304. [Google Scholar] [CrossRef]
- Blackwell, S. The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann. Clin. Biochem. 2010, 47, 17–28. [Google Scholar] [CrossRef]
- Luo, P.; Yan, M.; Frohlich, E.D.; Mehta, J.L.; Hu, C. Novel concepts in the genesis of hypertension: Role of LOX-1. Cardiovasc. Drugs Ther. 2011, 25, 441–449. [Google Scholar] [CrossRef]
- Devaraj, S.; Siegel, D.; Jialal, I. Statin therapy in metabolic syndrome and hypertension post-JUPITER: What is the value of CRP? Curr. Atheroscler. Rep. 2011, 13, 31–42. [Google Scholar] [CrossRef]
- Shih, H.H.; Zhang, S.; Cao, W.; Hahn, A.; Wang, J.; Paulsen, J.E.; Harnish, D.C. CRP is a novel ligand for the oxidized LDL receptor LOX-1. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1643–H1650. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Naska, A.; Orfanos, P.; Trichopoulos, D.; Mountokalakis, T.; Trichopoulou, A. Olive oil, the Mediterranean diet, and arterial blood pressure: The Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am. J. Clin. Nutr. 2004, 80, 1012–1018. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.R.; Hémon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Katan, M. Randomized clinical trials on the effects of dietary fat and carbohydrate on plasma lipoproteins and cardiovascular disease. Am. J. Med. 2002, 113 (Suppl. 9B), 13S–24S. [Google Scholar] [CrossRef]
- Roysommuti, S.; Khongnakha, T.; Jirakulsomchok, D.; Wyss, J.M. Excess dietary glucose alters renal function before increasing arterial pressure and inducing insulin resistance. Am. J. Hypertens. 2002, 15, 773–779. [Google Scholar] [CrossRef] [Green Version]
- Alonso, A.; Martínez-González, M.A. Olive oil consumption and reduced incidence of hypertension: The SUN study. Lipids 2004, 39, 1233–1238. [Google Scholar] [CrossRef] [PubMed]
- Perona, J.S.; Vögler, O.; Sánchez-Domínguez, J.M.; Montero, E.; Escribá, P.V.; Ruiz-Gutierrez, V. Consumption of virgin olive oil influences membrane lipid composition and regulates intracellular signaling in elderly adults with type 2 diabetes mellitus. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Gutiérrez, V.; Muriana, F.J.; Guerrero, A.; Cert, A.M.; Villar, J. Plasma lipids, erythrocyte membrane lipids and blood pressure of hypertensive women after ingestion of dietary oleic acid from two different sources. J. Hypertens. 1996, 14, 1483–1490. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Alemany, R.; Casas, J.; Kitajka, K.; Lanier, S.M.; Escribá, P.V. Influence of the membrane lipid structure on signal processing via G protein-coupled receptors. Mol. Pharmacol. 2005, 68, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Terés, S.; Baamonde, C.; Benet, M.; Vögler, O.; Escribá, P.V. 2-hydroxyoleic acid: A new hypotensive molecule. Hypertension 2004, 43, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Alemany, R.; Vögler, O.; Terés, S.; Egea, C.; Baamonde, C.; Barceló, F.; Delgado, C.; Jakobs, K.H.; Escribá, P.V. Antihypertensive action of 2-hydroxyoleic acid in SHRs via modulation of the protein kinase A pathway and Rho kinase. J. Lipid Res. 2006, 47, 1762–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visioli, F.; Galli, C. Antiatherogenic components of olive oil. Curr. Atheroscler. Rep. 2001, 3, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, L.A.; Raimondi, A.S.; d’Episcopo, L.; Guida, L.; Dello Russo, A.; Marotta, T. Olive oil and reduced need for antihypertensive medications. Arch. Intern. Med. 2000, 160, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; de Sotomayor, M.A.; Ruiz-Gutierrez, V. Pomace olive oil improves endothelial function in spontaneously hypertensive rats by increasing endothelial nitric oxide synthase expression. Am. J. Hypertens. 2007, 20, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, J.Y.; Holopainen, J.M.; Kinnunen, P.K. Evidence for the formation of microdomains in liquid crystalline large unilamellar vesicles caused by hydrophobic mismatch of the constituent phospholipids. Biophys. J. 1996, 70, 1753–1760. [Google Scholar] [CrossRef] [Green Version]
- Kinnunen, P.K.J. On the molecular-level mechanisms of peripheral protein-membrane interactions induced by lipids forming inverted non-lamellar phases. Chem. Phys. Lipids 1996, 81, 151–166. [Google Scholar] [CrossRef]
- Pérez, F.R.; Piñeiro, V.; De La Cruz, L.F.; Casanueva, F.F.; Casabiell, X. Vascular wall: Potential target for the physicochemical effects of cis-unsaturated free fatty acids. Microsc. Res. Tech. 2003, 60, 23–29. [Google Scholar] [CrossRef]
- Casabiell, X.; Pandiella, A.; Casanueva, F.F. Regulation of epidermal-growth-factor-receptor signal transduction by cis-unsaturated fatty acids. Evidence for a protein kinase C-independent mechanism. Biochem. J. 1991, 278, 679–687. [Google Scholar] [CrossRef]
- Doménech, M.; Roman, P.; Lapetra, J.; García de la Corte, F.J.; Sala-Vila, A.; de la Torre, R.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Lamuela-Raventós, R.-M.; et al. Mediterranean diet reduces 24-h ambulatory blood pressure, blood glucose, and lipids: One-year randomized, clinical trial. Hypertension 2014, 64, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Sheedy, F.; Fisher, E. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Colditz, G.A.; Rosner, B.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Frequent nut consumption and risk of coronary heart disease in women: Prospective cohort study. BMJ 1998, 317, 1341–1345. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Yu-Poth, S.; Sabaté, J.; Ratcliffe, H.E.; Zhao, G.; Etherton, T.D. Nuts and their bioactive constituents: Effects on serum lipids and other factors that affect disease risk. Am. J. Clin. Nutr. 1999, 70, 504S–511S. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Farrés, X.; Luque, X.; Narejos, S.; Borrell, M.; Basora, J.; Anguera, A.; Torres, F.; Bulló, M.; Balanza, R.; et al. Effect of two doses of a mixture of soluble fibres on body weight and metabolic variables in overweight or obese patients: A randomised trial. Br. J. Nutr. 2008, 99, 1380–1387. [Google Scholar] [CrossRef]
- Theuwissen, E.; Mensink, R.P. Water-soluble dietary fibers and cardiovascular disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef]
- Allaire, J.; Couture, P.; Leclerc, M.; Charest, A.; Marin, J.; Lépine, M.-C.; Talbot, D.; Tchernof, A.; Lamarche, B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: The Comparing EPA to DHA (ComparED) Study. Am. J. Clin. Nutr. 2016, 104, 280–287. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Skibsted, L.H. Carotenoids in antioxidant networks. Colorants or radical scavengers. J. Agric. Food Chem. 2012, 60, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Stephensen, C.B. Provitamin A Carotenoids and Immune Function. In Carotenoids and Human Health; Tanumihardjo, S.A., Ed.; Nutrition and Health; Humana Press: Totowa, NJ, USA, 2013; pp. 261–270. ISBN 978-1-62703-203-2. [Google Scholar]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Carotenoids: Potential allies of cardiovascular health? Food Nutr. Res. 2015, 59, 26762. [Google Scholar] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress--implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. N. Y. N 2014, 34, 907–929. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Simonen, P. Phytosterols, Phytostanols, and Lipoprotein Metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef] [PubMed]
- Richelle, M.; Enslen, M.; Hager, C.; Groux, M.; Tavazzi, I.; Godin, J.-P.; Berger, A.; Métairon, S.; Quaile, S.; Piguet-Welsch, C.; et al. Both free and esterified plant sterols reduce cholesterol absorption and the bioavailability of beta-carotene and alpha-tocopherol in normocholesterolemic humans. Am. J. Clin. Nutr. 2004, 80, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Gylling, H.; Plat, J.; Turley, S.; Ginsberg, H.N.; Ellegård, L.; Jessup, W.; Jones, P.J.; Lütjohann, D.; Maerz, W.; Masana, L.; et al. Plant sterols and plant stanols in the management of dyslipidaemia and prevention of cardiovascular disease. Atherosclerosis 2014, 232, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Zmitek, K.; Zmitek, J. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 2010, 50, 269–280. [Google Scholar] [CrossRef]
- Moss, J.W.E.; Ramji, D.P. Nutraceutical therapies for atherosclerosis. Nat. Rev. Cardiol. 2016, 13, 513–532. [Google Scholar] [CrossRef]
- Hernández-Camacho, J.D.; Bernier, M.; López-Lluch, G.; Navas, P. Coenzyme Q10 Supplementation in Aging and Disease. Front. Physiol. 2018, 9, 44. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-Y.; Yang, K.-L.; Zeng, L.-T.; Wu, X.-H.; Huang, H.-Y. Effectiveness of Coenzyme Q10 Supplementation for Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2018, 2018, 6484839. [Google Scholar] [CrossRef]
- Ambring, A.; Friberg, P.; Axelsen, M.; Laffrenzen, M.; Taskinen, M.-R.; Basu, S.; Johansson, M. Effects of a Mediterranean-inspired diet on blood lipids, vascular function and oxidative stress in healthy subjects. Clin. Sci. 2004, 106, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean Diet Improves High-Density Lipoprotein Function in High-Cardiovascular-Risk Individuals: A Randomized Controlled Trial. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Hovingh, G.K.; Hutten, B.A.; Holleboom, A.G.; Petersen, W.; Rol, P.; Stalenhoef, A.; Zwinderman, A.H.; de Groot, E.; Kastelein, J.J.P.; Kuivenhoven, J.A. Compromised LCAT function is associated with increased atherosclerosis. Circulation 2005, 112, 879–884. [Google Scholar] [CrossRef]
- Wang, K.; Subbaiah, P.V. Importance of the free sulfhydryl groups of lecithin-cholesterol acyltransferase for its sensitivity to oxidative inactivation. Biochim. Biophys. Acta 2000, 1488, 268–277. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B.; Ansell, B.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Translation of high-density lipoprotein function into clinical practice: Current prospects and future challenges. Circulation 2013, 128, 1256–1267. [Google Scholar] [CrossRef]
- Ansell, B.J.; Navab, M.; Hama, S.; Kamranpour, N.; Fonarow, G.; Hough, G.; Rahmani, S.; Mottahedeh, R.; Dave, R.; Reddy, S.T.; et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-density lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 2003, 108, 2751–2756. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Hartiala, J.; Fan, Y.; Wu, Y.; Stewart, A.F.R.; Erdmann, J.; Kathiresan, S.; CARDIoGRAM Consortium; Roberts, R.; McPherson, R.; et al. Clinical and genetic association of serum paraoxonase and arylesterase activities with cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2803–2812. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Serum paraoxonase-1 activity and risk of incident cardiovascular disease: The PREVEND study and meta-analysis of prospective population studies. Atherosclerosis 2016, 245, 143–154. [Google Scholar] [CrossRef]
- Besler, C.; Lüscher, T.F.; Landmesser, U. Molecular mechanisms of vascular effects of High-density lipoprotein: Alterations in cardiovascular disease. EMBO Mol. Med. 2012, 4, 251–268. [Google Scholar] [CrossRef]
- Tuccinardi, D.; Farr, O.M.; Upadhyay, J.; Oussaada, S.M.; Klapa, M.I.; Candela, M.; Rampelli, S.; Lehoux, S.; Lázaro, I.; Sala-Vila, A.; et al. Mechanisms underlying the cardiometabolic protective effect of walnut consumption in obese people: A cross-over, randomized, double-blind, controlled inpatient physiology study. Diabetes Obes. Metab. 2019, 21, 2086–2095. [Google Scholar] [CrossRef]
- Chavez, J.A.; Summers, S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012, 15, 585–594. [Google Scholar] [CrossRef]
- El Harchaoui, K.; Arsenault, B.J.; Franssen, R.; Després, J.-P.; Hovingh, G.K.; Stroes, E.S.G.; Otvos, J.D.; Wareham, N.J.; Kastelein, J.J.P.; Khaw, K.-T.; et al. High-density lipoprotein particle size and concentration and coronary risk. Ann. Intern. Med. 2009, 150, 84–93. [Google Scholar] [CrossRef]
- U.S. Food & Drug. Qualified Health Claims: Letters of Enforcement Discretion. 2019. Available online: https://www.fda.gov/food/food-labeling-nutrition/qualified-health-claims-letters-enforcement-discretion (accessed on 19 June 2009).
- Sala-Vila, A.; Cofán, M.; Núñez, I.; Gilabert, R.; Junyent, M.; Ros, E. Carotid and femoral plaque burden is inversely associated with the α-linolenic acid proportion of serum phospholipids in Spanish subjects with primary dyslipidemia. Atherosclerosis 2011, 214, 209–214. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention with Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef]
- Gepner, Y.; Shelef, I.; Komy, O.; Cohen, N.; Schwarzfuchs, D.; Bril, N.; Rein, M.; Serfaty, D.; Kenigsbuch, S.; Zelicha, H.; et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J. Hepatol. 2019, 71, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Schröder, H.; Marrugat, J.; Elosua, R.; Covas, M.I. REGICOR Investigators Relationship between body mass index, serum cholesterol, leisure-time physical activity, and diet in a Mediterranean Southern-Europe population. Br. J. Nutr. 2003, 90, 431–439. [Google Scholar]
- Tzima, N.; Pitsavos, C.; Panagiotakos, D.B.; Skoumas, J.; Zampelas, A.; Chrysohoou, C.; Stefanadis, C. Mediterranean diet and insulin sensitivity, lipid profile and blood pressure levels, in overweight and obese people; the Attica study. Lipids Health Dis. 2007, 6, 22. [Google Scholar] [CrossRef]
- Summers, S.A. Ceramides in insulin resistance and lipotoxicity. Prog. Lipid Res. 2006, 45, 42–72. [Google Scholar] [CrossRef]
- Summers, S.A. The ART of Lowering Ceramides. Cell Metab. 2015, 22, 195–196. [Google Scholar] [CrossRef] [Green Version]
- Havulinna, A.S.; Sysi-Aho, M.; Hilvo, M.; Kauhanen, D.; Hurme, R.; Ekroos, K.; Salomaa, V.; Laaksonen, R. Circulating Ceramides Predict Cardiovascular Outcomes in the Population-Based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2424–2430. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Bonapace, S.; Lunardi, G.; Salgarello, M.; Dugo, C.; Canali, G.; Byrne, C.D.; Gori, S.; Barbieri, E.; Targher, G. Association between plasma ceramides and inducible myocardial ischemia in patients with established or suspected coronary artery disease undergoing myocardial perfusion scintigraphy. Metabolism. 2018, 85, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Ros, E.; Martínez-González, M.A.; Estruch, R.; Salas-Salvadó, J.; Fitó, M.; Martínez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the PREDIMED study. Adv. Nutr. Bethesda Md 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Toledo, E.; Arós, F.; Fiol, M.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fernández-Crehuet, J.; Lapetra, J.; et al. Extravirgin olive oil consumption reduces risk of atrial fibrillation: The PREDIMED (Prevención con Dieta Mediterránea) trial. Circulation 2014, 130, 18–26. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; de la Torre, R.; Francés, F.; Cabezas, C.; López-Sabater, M.D.C.; Marrugat, J.; et al. Effect of a traditional Mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106, S5–S78. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Ogiwara, T.; Satoh, K.; Kadoma, Y.; Murakami, Y.; Unten, S.; Atsumi, T.; Sakagami, H.; Fujisawa, S. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 2002, 22, 2711–2717. [Google Scholar]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Kouli, G.-M.; Panagiotakos, D.B.; Kyrou, I.; Magriplis, E.; Georgousopoulou, E.N.; Chrysohoou, C.; Tsigos, C.; Tousoulis, D.; Pitsavos, C. Olive oil consumption and 10-year (2002–2012) cardiovascular disease incidence: The ATTICA study. Eur. J. Nutr. 2019, 58, 131–138. [Google Scholar] [CrossRef]
- Urquiaga, I.; Strobel, P.; Perez, D.; Martinez, C.; Cuevas, A.; Castillo, O.; Marshall, G.; Rozowski, J.; Leighton, F. Mediterranean diet and red wine protect against oxidative damage in young volunteers. Atherosclerosis 2010, 211, 694–699. [Google Scholar] [CrossRef]
- Wu, L.L.; Chiou, C.C.; Chang, P.Y.; Wu, J.T. Urinary 8-OHdG: A marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin. Chim. Acta Int. J. Clin. Chem. 2004, 339, 1–9. [Google Scholar] [CrossRef]
- Quintero-Flórez, A.; Sinausia Nieva, L.; Sánchez-Ortíz, A.; Beltrán, G.; Perona, J.S. The Fatty Acid Composition of Virgin Olive Oil from Different Cultivars Is Determinant for Foam Cell Formation by Macrophages. J. Agric. Food Chem. 2015, 63, 6731–6738. [Google Scholar] [CrossRef]
- Varela, L.M.; Ortega-Gomez, A.; Lopez, S.; Abia, R.; Muriana, F.J.G.; Bermudez, B. The effects of dietary fatty acids on the postprandial triglyceride-rich lipoprotein/apoB48 receptor axis in human monocyte/macrophage cells. J. Nutr. Biochem. 2013, 24, 2031–2039. [Google Scholar] [CrossRef]
- Vallvé, J.-C.; Uliaque, K.; Girona, J.; Cabré, A.; Ribalta, J.; Heras, M.; Masana, L. Unsaturated fatty acids and their oxidation products stimulate CD36 gene expression in human macrophages. Atherosclerosis 2002, 164, 45–56. [Google Scholar] [CrossRef]
- Endemann, G.; Stanton, L.W.; Madden, K.S.; Bryant, C.M.; White, R.T.; Protter, A.A. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 1993, 268, 11811–11816. [Google Scholar]
- Tontonoz, P.; Nagy, L.; Alvarez, J.G.; Thomazy, V.A.; Evans, R.M. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 1998, 93, 241–252. [Google Scholar] [CrossRef]
- Tontonoz, P.; Nagy, L. Regulation of macrophage gene expression by peroxisome-proliferator-activated receptor gamma: Implications for cardiovascular disease. Curr. Opin. Lipidol. 1999, 10, 485–490. [Google Scholar] [CrossRef]
- Mancia, G.; Laurent, S.; Agabiti-Rosei, E.; Ambrosioni, E.; Burnier, M.; Caulfield, M.J.; Cifkova, R.; Clément, D.; Coca, A.; Dominiczak, A.; et al. Reappraisal of European guidelines on hypertension management: A European Society of Hypertension Task Force document. J. Hypertens. 2009, 27, 2121–2158. [Google Scholar] [CrossRef]
- Jung, J.Y.; Hwang, Y.-H.; Lee, H.; Ro, H.; Lee, H.; Chung, W.; Chae, D.-W.; Joo, K.W.; Ahn, C.; Oh, K.-H. Association of AHSG gene polymorphisms and aortic stiffness in peritoneal dialysis patients. Am. J. Nephrol. 2010, 31, 510–517. [Google Scholar] [CrossRef]
- Iemitsu, M.; Murakami, H.; Sanada, K.; Yamamoto, K.; Kawano, H.; Gando, Y.; Miyachi, M. Lack of carotid stiffening associated with MTHFR 677TT genotype in cardiorespiratory fit adults. Physiol. Genom. 2010, 42, 259–265. [Google Scholar] [CrossRef] [Green Version]
- Van de Laar, R.J.J.; Stehouwer, C.D.A.; van Bussel, B.C.T.; Prins, M.H.; Twisk, J.W.R.; Ferreira, I. Adherence to a Mediterranean dietary pattern in early life is associated with lower arterial stiffness in adulthood: The Amsterdam Growth and Health Longitudinal Study. J. Intern. Med. 2013, 273, 79–93. [Google Scholar] [CrossRef]
- Rodríguez-Martin, C.; Alonso-Domínguez, R.; Patino-Alonso, M.C.; Gómez-Marcos, M.A.; Maderuelo-Fernández, J.A.; Martin-Cantera, C.; García-Ortiz, L.; Recio-Rodríguez, J.I. EVIDENT group The EVIDENT diet quality index is associated with cardiovascular risk and arterial stiffness in adults. BMC Public Health 2017, 17, 305. [Google Scholar]
- Giugliano, D.; Ceriello, A.; Esposito, K. The effects of diet on inflammation: Emphasis on the metabolic syndrome. J. Am. Coll. Cardiol. 2006, 48, 677–685. [Google Scholar] [CrossRef]
- Shai, I.; Spence, J.D.; Schwarzfuchs, D.; Henkin, Y.; Parraga, G.; Rudich, A.; Fenster, A.; Mallett, C.; Liel-Cohen, N.; Tirosh, A.; et al. Dietary intervention to reverse carotid atherosclerosis. Circulation 2010, 121, 1200–1208. [Google Scholar] [CrossRef]
- Murie-Fernandez, M.; Irimia, P.; Toledo, E.; Martínez-Vila, E.; Buil-Cosiales, P.; Serrano-Martínez, M.; Ruiz-Gutiérrez, V.; Ros, E.; Estruch, R.; Martínez-González, M.Á.; et al. Carotid intima-media thickness changes with Mediterranean diet: A randomized trial (PREDIMED-Navarra). Atherosclerosis 2011, 219, 158–162. [Google Scholar] [CrossRef]
- Akgüllü, Ç.; Sırıken, F.; Eryılmaz, U.; Akdeniz, M.; Ömürlü, İ.K.; Pekcan, G.; Güngör, H.; Kurtoğlu, T. The relation between compliance to the Mediterranean diet and the extensiveness of coronary artery disease. Turk. Kardiyol. Dern. Ars. Turk. Kardiyol. Dern. Yayin Organidir 2015, 43, 340–349. [Google Scholar]
- Waldeyer, C.; Brunner, F.J.; Braetz, J.; Ruebsamen, N.; Zyriax, B.-C.; Blaum, C.; Kroeger, F.; Kohsiack, R.; Schrage, B.; Sinning, C.; et al. Adherence to Mediterranean diet, high-sensitive C-reactive protein, and severity of coronary artery disease: Contemporary data from the INTERCATH cohort. Atherosclerosis 2018, 275, 256–261. [Google Scholar] [CrossRef]
- Acar, B.; Gucuk Ipek, E.; Unal, S.; Yayla, C.; Karanfil, M.; Burak, C.; Kara, M.; Bayraktar, F.; Kuyumcu, M.S.; Aydogdu, S. Evaluation of Mediterranean diet adherence in patients with a history of coronary revascularization. Rev. Clin. Esp. 2018, 218, 215–222. [Google Scholar] [CrossRef]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Chrysohoou, C.; Pitsavos, C.; Tzioumis, K.; Papaioannou, I.; Stefanadis, C.; Toutouzas, P. The association of Mediterranean diet with lower risk of acute coronary syndromes in hypertensive subjects. Int. J. Cardiol. 2002, 82, 141–147. [Google Scholar] [CrossRef]
- Avery, C.L.; Loehr, L.R.; Baggett, C.; Chang, P.P.; Kucharska-Newton, A.M.; Matsushita, K.; Rosamond, W.D.; Heiss, G. The population burden of heart failure attributable to modifiable risk factors: The ARIC (Atherosclerosis Risk in Communities) study. J. Am. Coll. Cardiol. 2012, 60, 1640–1646. [Google Scholar] [CrossRef]
- Sanches Machado d’Almeida, K.; Ronchi Spillere, S.; Zuchinali, P.; Corrêa Souza, G. Mediterranean Diet and Other Dietary Patterns in Primary Prevention of Heart Failure and Changes in Cardiac Function Markers: A Systematic Review. Nutrients 2018, 10, 58. [Google Scholar] [CrossRef]
- He, J.; Ogden, L.G.; Bazzano, L.A.; Vupputuri, S.; Loria, C.; Whelton, P.K. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch. Intern. Med. 2001, 161, 996–1002. [Google Scholar] [CrossRef]
- Heckbert, S.R.; Post, W.; Pearson, G.D.N.; Arnett, D.K.; Gomes, A.S.; Jerosch-Herold, M.; Hundley, W.G.; Lima, J.A.; Bluemke, D.A. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: The Multiethnic Study of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 48, 2285–2292. [Google Scholar] [CrossRef]
- Papadaki, A.; Martínez-González, M.Á.; Alonso-Gómez, A.; Rekondo, J.; Salas-Salvadó, J.; Corella, D.; Ros, E.; Fitó, M.; Estruch, R.; Lapetra, J.; et al. Mediterranean diet and risk of heart failure: Results from the PREDIMED randomized controlled trial. Eur. J. Heart Fail. 2017, 19, 1179–1185. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Albert, N.M.; Allen, L.A.; Bluemke, D.A.; Butler, J.; Fonarow, G.C.; Ikonomidis, J.S.; Khavjou, O.; Konstam, M.A.; Maddox, T.M.; et al. Forecasting the impact of heart failure in the United States: A policy statement from the American Heart Association. Circ. Heart Fail. 2013, 6, 606–619. [Google Scholar] [CrossRef]
- Dai, J.; Jones, D.P.; Goldberg, J.; Ziegler, T.R.; Bostick, R.M.; Wilson, P.W.; Manatunga, A.K.; Shallenberger, L.; Jones, L.; Vaccarino, V. Association between adherence to the Mediterranean diet and oxidative stress. Am. J. Clin. Nutr. 2008, 88, 1364–1370. [Google Scholar]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef] [Green Version]
- Chrysohoou, C.; Pitsavos, C.; Barbetseas, J.; Kotroyiannis, I.; Brili, S.; Vasiliadou, K.; Papadimitriou, L.; Stefanadis, C. Chronic systemic inflammation accompanies impaired ventricular diastolic function, detected by Doppler imaging, in patients with newly diagnosed systolic heart failure (Hellenic Heart Failure Study). Heart Vessels 2009, 24, 22–26. [Google Scholar] [CrossRef]
- Fitó, M.; Estruch, R.; Salas-Salvadó, J.; Martínez-Gonzalez, M.A.; Arós, F.; Vila, J.; Corella, D.; Díaz, O.; Sáez, G.; de la Torre, R.; et al. Effect of the Mediterranean diet on heart failure biomarkers: A randomized sample from the PREDIMED trial. Eur. J. Heart Fail. 2014, 16, 543–550. [Google Scholar] [CrossRef]
- Brouwers, F.P.; de Boer, R.A.; van der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; van Veldhuisen, D.J.; van Gilst, W.H. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef]
- Kistorp, C.; Raymond, I.; Pedersen, F.; Gustafsson, F.; Faber, J.; Hildebrandt, P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 2005, 293, 1609–1616. [Google Scholar] [CrossRef]
- Meirovich, Y.F.; Veinot, J.P.; de Bold, M.L.K.; Haddad, H.; Davies, R.A.; Masters, R.G.; Hendry, P.J.; de Bold, A.J. Relationship between natriuretic peptides and inflammation: Proteomic evidence obtained during acute cellular cardiac allograft rejection in humans. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2008, 27, 31–37. [Google Scholar] [CrossRef]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Salas-Salvadó, J.; Covas, M.I.; Toledo, E.; Andres-Lacueva, C.; Llorach, R.; et al. The Mediterranean diet pattern and its main components are associated with lower plasma concentrations of tumor necrosis factor receptor 60 in patients at high risk for cardiovascular disease. J. Nutr. 2012, 142, 1019–1025. [Google Scholar] [CrossRef]
- Yubero-Serrano, E.M.; Gonzalez-Guardia, L.; Rangel-Zuñiga, O.; Delgado-Lista, J.; Gutierrez-Mariscal, F.M.; Perez-Martinez, P.; Delgado-Casado, N.; Cruz-Teno, C.; Tinahones, F.J.; Villalba, J.M.; et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J. Gerontol. A. Biol. Sci. Med. Sci. 2012, 67, 3–10. [Google Scholar] [CrossRef]
- Belch, J.J.; Bridges, A.B.; Scott, N.; Chopra, M. Oxygen free radicals and congestive heart failure. Br. Heart J. 1991, 65, 245–248. [Google Scholar] [CrossRef]
- Hill, M.F.; Singal, P.K. Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am. J. Pathol. 1996, 148, 291–300. [Google Scholar]
- Sawyer, D.B.; Colucci, W.S. Mitochondrial oxidative stress in heart failure: “oxygen wastage” revisited. Circ. Res. 2000, 86, 119–120. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Hayashidani, S.; Kang, D.; Suematsu, N.; Nakamura, K.; Utsumi, H.; Hamasaki, N.; Takeshita, A. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ. Res. 2001, 88, 529–535. [Google Scholar] [CrossRef]
- Sabri, A.; Hughie, H.H.; Lucchesi, P.A. Regulation of hypertrophic and apoptotic signaling pathways by reactive oxygen species in cardiac myocytes. Antioxid. Redox Signal. 2003, 5, 731–740. [Google Scholar] [CrossRef]
- Cesselli, D.; Jakoniuk, I.; Barlucchi, L.; Beltrami, A.P.; Hintze, T.H.; Nadal-Ginard, B.; Kajstura, J.; Leri, A.; Anversa, P. Oxidative stress-mediated cardiac cell death is a major determinant of ventricular dysfunction and failure in dog dilated cardiomyopathy. Circ. Res. 2001, 89, 279–286. [Google Scholar] [CrossRef]
- Spinale, F.G.; Coker, M.L.; Thomas, C.V.; Walker, J.D.; Mukherjee, R.; Hebbar, L. Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: Relation to ventricular and myocyte function. Circ. Res. 1998, 82, 482–495. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Meng, X.P.; Ramasamy, S.; Harrison, D.G.; Galis, Z.S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Investig. 1996, 98, 2572–2579. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. The role of antioxidants in the Mediterranean diet. Lipids 2001, 36, S49–S52. [Google Scholar] [CrossRef]
- Álvarez, P.; Alvarado, C.; Mathieu, F.; Jiménez, L.; De la Fuente, M. Diet supplementation for 5 weeks with polyphenol-rich cereals improves several functions and the redox state of mouse leucocytes. Eur. J. Nutr. 2006, 45, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Davis, L.; Stonehouse, W.; Loots, D.T.; Mukuddem-Petersen, J.; van der Westhuizen, F.H.; Hanekom, S.M.; Jerling, J.C. The effects of high walnut and cashew nut diets on the antioxidant status of subjects with metabolic syndrome. Eur. J. Nutr. 2007, 46, 155–164. [Google Scholar] [CrossRef]
- Le, C.T.; Hollaar, L.; Van der Valk, E.J.; Franken, N.A.; Van Ravels, F.J.; Wondergem, J.; Van der Laarse, A. Protection of myocytes against free radical-induced damage by accelerated turnover of the glutathione redox cycle. Eur. Heart J. 1995, 16, 553–562. [Google Scholar] [CrossRef]
- Fico, A.; Paglialunga, F.; Cigliano, L.; Abrescia, P.; Verde, P.; Martini, G.; Iaccarino, I.; Filosa, S. Glucose-6-phosphate dehydrogenase plays a crucial role in protection from redox-stress-induced apoptosis. Cell Death Differ. 2004, 11, 823–831. [Google Scholar] [CrossRef]
- Fang, Y.-Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutr. Burbank Los Angel. Cty. Calif 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Rebrin, I.; Zicker, S.; Wedekind, K.J.; Paetau-Robinson, I.; Packer, L.; Sohal, R.S. Effect of antioxidant-enriched diets on glutathione redox status in tissue homogenates and mitochondria of the senescence-accelerated mouse. Free Radic. Biol. Med. 2005, 39, 549–557. [Google Scholar] [CrossRef] [Green Version]
- Yeung, A.W.K.; Aggarwal, B.B.; Orhan, I.E.; Horbanczuk, O.K.; Barreca, D.; Battino, M.; Belwal, T.; Bishayee, A.; Daglia, M.; Devkota, H.P.; et al. Resveratrol, a popular dietary supplement for human and animal health: Quantitative research literature analysis—A review. Anim. Sci. Pap. Rep. 2019. [Google Scholar]
- Tsutsui, T.; Tsutamoto, T.; Wada, A.; Maeda, K.; Mabuchi, N.; Hayashi, M.; Ohnishi, M.; Kinoshita, M. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J. Am. Coll. Cardiol. 2002, 39, 957–962. [Google Scholar] [CrossRef] [Green Version]
- Charach, G.; George, J.; Afek, A.; Wexler, D.; Sheps, D.; Keren, G.; Rubinstein, A. Antibodies to oxidized LDL as predictors of morbidity and mortality in patients with chronic heart failure. J. Card. Fail. 2009, 15, 770–774. [Google Scholar] [CrossRef]
- Bergmark, C.; Dewan, A.; Orsoni, A.; Merki, E.; Miller, E.R.; Shin, M.-J.; Binder, C.J.; Hörkkö, S.; Krauss, R.M.; Chapman, M.J.; et al. A novel function of lipoprotein as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008, 49, 2230–2239. [Google Scholar] [CrossRef]
- Shin, M.-J.; Blanche, P.J.; Rawlings, R.S.; Fernstrom, H.S.; Krauss, R.M. Increased plasma concentrations of lipoprotein(a) during a low-fat, high-carbohydrate diet are associated with increased plasma concentrations of apolipoprotein C-III bound to apolipoprotein B-containing lipoproteins. Am. J. Clin. Nutr. 2007, 85, 1527–1532. [Google Scholar] [CrossRef]
- Perona, J.S.; Covas, M.-I.; Fitó, M.; Cabello-Moruno, R.; Aros, F.; Corella, D.; Ros, E.; Garcia, M.; Estruch, R.; Martinez-Gonzalez, M.A.; et al. Reduction in systemic and VLDL triacylglycerol concentration after a 3-month Mediterranean-style diet in high-cardiovascular-risk subjects. J. Nutr. Biochem. 2010, 21, 892–898. [Google Scholar] [CrossRef]
- Willett, W.C.; Stampfer, M.J. Rebuilding the food pyramid. Sci. Am. 2003, 288, 64–71. [Google Scholar] [CrossRef]
- Singh, R.B.; Dubnov, G.; Niaz, M.A.; Ghosh, S.; Singh, R.; Rastogi, S.S.; Manor, O.; Pella, D.; Berry, E.M. Effect of an Indo-Mediterranean diet on progression of coronary artery disease in high risk patients (Indo-Mediterranean Diet Heart Study): A randomised single-blind trial. Lancet Lond. Engl. 2002, 360, 1455–1461. [Google Scholar] [CrossRef]
- Chen, G.-C.; Neelakantan, N.; Martín-Calvo, N.; Koh, W.-P.; Yuan, J.-M.; Bonaccio, M.; Iacoviello, L.; Martínez-González, M.A.; Qin, L.-Q.; van Dam, R.M. Adherence to the Mediterranean diet and risk of stroke and stroke subtypes. Eur. J. Epidemiol. 2019, 34, 337–349. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet Lond. Engl. 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Bendinelli, B.; Masala, G.; Saieva, C.; Salvini, S.; Calonico, C.; Sacerdote, C.; Agnoli, C.; Grioni, S.; Frasca, G.; Mattiello, A.; et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: The EPICOR Study. Am. J. Clin. Nutr. 2011, 93, 275–283. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Fernández-Ballart, J.; Ros, E.; Martínez-González, M.-A.; Fitó, M.; Estruch, R.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Arós, F.; et al. Effect of a Mediterranean diet supplemented with nuts on metabolic syndrome status: One-year results of the PREDIMED randomized trial. Arch. Intern. Med. 2008, 168, 2449–2458. [Google Scholar] [CrossRef]
- Kastorini, C.-M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The effect of Mediterranean diet on metabolic syndrome and its components: A meta-analysis of 50 studies and 534,906 individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ceriello, A.; Giugliano, D. Prevention and control of type 2 diabetes by Mediterranean diet: A systematic review. Diabetes Res. Clin. Pract. 2010, 89, 97–102. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet Lond. Engl. 2012, 380, 581–590. [Google Scholar]
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet Lond. Engl. 1994, 344, 1383–1389.
- Tuttolomondo, A.; Casuccio, A.; Buttà, C.; Pecoraro, R.; Di Raimondo, D.; Della Corte, V.; Arnao, V.; Clemente, G.; Maida, C.; Simonetta, I.; et al. Mediterranean Diet in patients with acute ischemic stroke: Relationships between Mediterranean Diet score, diagnostic subtype, and stroke severity index. Atherosclerosis 2015, 243, 260–267. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome as a possible cardiovascular marker in diabetic patients. J. Diabetes Res. 2015, 2015, 268390. [Google Scholar] [CrossRef]
- Siragusa, S.; Malato, A.; Saccullo, G.; Iorio, A.; Di Ianni, M.; Caracciolo, C.; Coco, L.L.; Raso, S.; Santoro, M.; Guarneri, F.P.; et al. Residual vein thrombosis for assessing duration of anticoagulation after unprovoked deep vein thrombosis of the lower limbs: The extended DACUS study. Am. J. Hematol. 2011, 86, 914–917. [Google Scholar] [CrossRef] [Green Version]
- Conte, G.; Rigon, N.; Perrone, A.; Lauro, S. Acute cardiovascular diseases and respiratory sleep disorders. Minerva Cardioangiol. 1999, 47, 195–202. [Google Scholar]


| Author and Year | Brief Description | Conclusions |
|---|---|---|
| Due, A. et al. 2008 [81] | 46 nondiabetic, obese men (20) and premenopausal women (26) randomly assigned to 1 of 3 diets:
| A diet high in monounsaturated fat has a more favourable effect on glucose homeostasis than does the typical Western diet in the short term and may also be more beneficial than the official recommended low-fat diet during a period of weight regain subsequent to weight loss. |
| Paniagua, J.A. et al. 2007 [82] | A prospective study performed in eleven (7 W, 4M) offspring of obese and type 2 diabetes patients randomly divided into three groups and underwent three dietary periods each of 28 days in a crossover design:
| Weight maintenance with a MUFA- rich diet improves HOMA-ir and fasting proinsulin levels in insulin- resistant subjects. Ingestion of a virgin olive oil-based breakfast decreased postprandial glucose and insulin conc entrations, and increased HDL-C and GLP-1 concentrations as compared with CHO-rich diet |
| Shah, M. et al. 2007 [91] | Test meals rich in palmitic acid, linoleic acid, oleic acid, and eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and containing 1000 kcal each were administered in a randomized crossover design to 11 type 2 diabetic subjects | In comparison with palmitic acid and linoleic acid, oleic acid or EPA and DHA may modestly lower insulin response in patients with type 2 diabetes without deteriorating the glucose response. EPA and DHA may also reduce the triglyceride response |
| Perez-Jimenez, F. et al. 2001 [92] | Intervention dietary study with a saturated fat phase and two randomized-crossover dietary periods: a high-carbohydrate diet and a Mediterranean diet for 28 days each | Isocaloric substitution of carbohydrates and monounsaturated fatty acids for saturated fatty acids improved insulin sensitivity in vivo and in vitro, with an increase in glucose disposal. Both diets are an adequate alternatives for improving glucose metabolism in healthy young men and women. |
| Brehm, B.J. et al. 2009 [89] | Overweight/obese participants with type 2 diabetes (n = 124, age = 56.5 ± 0.8 years, BMI = 35.9 ± 0.3 kg/m2, and A1C = 7.3 ± 0.1%) were randomly assigned to 1 year of a high-MUFA or high-CHO diet | In individuals with type 2 diabetes, high-MUFA diets are an alternative to conventional lower-fat, high-CHO diets with comparable beneficial effects on body weight, body composition, cardiovascular risk factors, and glycemic control |
| Vessby, B. et al. 2001 [83] | The KANWU study included 162 healthy subjects chosen at random to receive a controlled, isoenergetic diet for 3 months containing either a high proportion of saturated (SAFA diet) or monounsaturated (MUFA diet) fatty acids. Within each group there was a second assignment at random to supplements with fish oil (3.6 g n-3 fatty acids/d) or placebo | A change of the proportions of dietary fatty acids, decreasing saturated fatty acid and increasing monounsaturated fatty acid, improves insulin sensitivity but has no effect on insulin secretion. A beneficial impact of the fat quality on insulin sensitivity is not seen in individuals with a high fat intake |
| Author and Journal | Brief Description | Conclusions |
|---|---|---|
| Toledo et al. 2013 [99] | The PREDIMED primary prevention trial is a randomized, single-blinded, controlled trial conducted in Spanish primary healthcare centers. 7447 men (aged 55 to 80 years) and women (aged 60 to 80 years) who had high risk for cardiovascular disease were assigned to a control group or to one of two Mediterranean diets. The control group received education on following a low-fat diet, while the groups on Mediterranean diets received nutritional education and also free foods; either extra virgin olive oil, or nuts. | Both the traditional Mediterranean diet and a low-fat diet exerted beneficial effects on BP and could be part of advice to patients for controlling BP. However, we found lower values of diastolic BP in the two groups promoting the Mediterranean diet with extra virgin olive oil or with nuts than in the control group. |
| Storniolo et al. 2017 [105] | Non-smoking women with moderate hypertension were submitted for 1 year to interventions promoting adherence to the TMD, one supplemented with extra virgin olive oil (EVOO) and the other with nuts versus a control low-fat diet (30 participants/group). BP, NO, ET-1 and related gene expression as well as oxidative stress biomarkers were measured. | The changes in NO and ET-1 as well as ET-1 receptors gene expression explain, at least partially, the effect of EVOO or nuts on lowering BP among hypertensive women. |
| Nissensohn et al. 2016 [98] | Six trials (more than 7000 individuals) were identified. Meta-analysis showed that interventions aiming at adopting an MD pattern for at least 1 year reduced both the systolic BP and diastolic BP levels in individuals with normal BP or mild hypertension. | A positive and significant association was found between the MD and BP in adults. |
| Moreno-Luna et al. 2012 [112] | Double-blind, randomized, crossover dietary- intervention study. After a run-in period of 4 months (baseline values), two diets were used, one with polyphenol-rich olive oil (∼30 mg/day), the other with polyphenol-free olive oil. Each dietary period lasted 2 months with a 4-week washout between diets | The consumption of a diet containing polyphenol-rich olive oil can decrease BP and improve endothelial function in young women with high-normal BP or stage 1 essential hypertension. |
| Alonso et al. 2004 [122] | Prospective cohort study whose members are all university graduates to assess the risk of hypertension associated with olive oil consumption. | In a Mediterranean population, we found olive oil consumption to be associated with a reduced risk of hypertension only among men. |
| Psaltopoulou et al. 2004 [117] | Arterial blood pressure and several sociodemographic, anthropometric, dietary, physical activity, and clinical variables were recorded at enrollment among participants in the Greek arm of the European Prospective Investigation into Cancer and Nutrition (EPIC) study. | Adherence to the Mediterranean diet is inversely associated with arterial blood pressure, even though a beneficial component of the Mediterranean diet score-cereal intake-is positively associated with arterial blood pressure. Olive oil intake, per se, is inversely associated with both systolic and diastolic blood pressure. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194716
Tuttolomondo A, Simonetta I, Daidone M, Mogavero A, Ortello A, Pinto A. Metabolic and Vascular Effect of the Mediterranean Diet. International Journal of Molecular Sciences. 2019; 20(19):4716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194716
Chicago/Turabian StyleTuttolomondo, Antonino, Irene Simonetta, Mario Daidone, Alba Mogavero, Antonella Ortello, and Antonio Pinto. 2019. "Metabolic and Vascular Effect of the Mediterranean Diet" International Journal of Molecular Sciences 20, no. 19: 4716. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194716