Interferon-Inducible Protein 10 and Disease Activity in Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
2.1. Study Characteristics
2.2. Quality Assessment
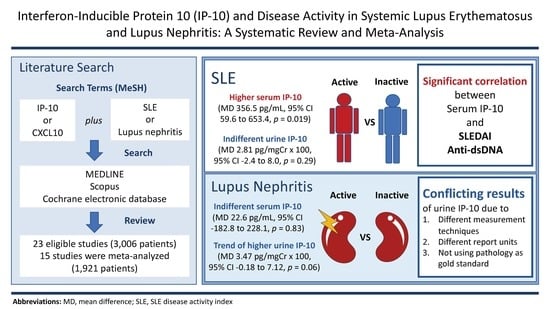
2.3. Serum IP-10 and SLE
2.4. Serum IP-10 and LN
2.5. Urine IP-10 and SLE
2.6. Urine IP-10 and LN
2.7. Serum IP-10 vs urine IP-10
3. Discussion
4. Materials and Methods
4.1. Literature Search
4.2. Study Selection Criteria
4.3. Data Extraction and Quality Assessment
4.4. Data Synthesis and Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CBA | cytometric bead array |
| ELISA | enzyme-linked immunosorbent assay |
| IP-10 | interferon-inducible protein 10 |
| LN | lupus nephritis |
| mRNA | messenger RNA |
| NR | not reported |
| qRT-PCR | quantitative reverse transcription-polymerase chain reaction |
| SLE | systemic lupus erythematosus. |
References
- Ho, A.; Barr, S.G.; Magder, L.S.; Petri, M. A decrease in complement is associated with increased renal and hematologic activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2001, 44, 2350–2357. [Google Scholar] [CrossRef]
- Julkunen, H.; Ekblom-Kullberg, S.; Miettinen, A. Nonrenal and renal activity of systemic lupus erythematosus: A comparison of two anti-C1q and five anti-dsDNA assays and complement C3 and C4. Rheumatol. Int. 2012, 32, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Schnitzer, T.J. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Hooks, J.J.; Moutsopoulos, H.M.; Geis, S.A.; Stahl, N.I.; Decker, J.L.; Notkins, A.L. Immune Interferon in the Circulation of Patients with Autoimmune Disease. N. Engl. J. Med. 1979, 301, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Gota, C.; Calabrese, L. Induction of clinical autoimmune disease by therapeutic interferon-alpha. Autoimmunity 2003, 36, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.; Palucka, A.K.; Arce, E.; Cantrell, V.; Borvak, J.; Banchereau, J.; Pascual, V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003, 197, 711–723. [Google Scholar] [CrossRef]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, J.W.; Baechler, E.C.; Petri, M.; Batliwalla, F.M.; Crawford, D.; Ortmann, W.A.; Espe, K.J.; Li, W.; Patel, D.D.; Gregersen, P.K.; et al. Elevated serum levels of interferon-regulated chemokines are biomarkers for active human systemic lupus erythematosus. PLoS Med. 2006, 3, e491. [Google Scholar] [CrossRef]
- Shiozawa, F.; Kasama, T.; Yajima, N.; Odai, T.; Isozaki, T.; Matsunawa, M.; Yoda, Y.; Negishi, M.; Ide, H.; Adachi, M. Enhanced expression of interferon-inducible protein 10 associated with Th1 profiles of chemokine receptor in autoimmune pulmonary inflammation of MRL/lprmice. Arthritis Res. Ther. 2003, 6, R78. [Google Scholar] [CrossRef]
- Lu, J.; Kwan, B.C.; Lai, F.M.; Choi, P.C.; Tam, L.S.; Li, E.K.; Chow, K.M.; Wang, G.; Li, P.K.; Szeto, C.C. Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrol. (CarltonVic.) 2011, 16, 426–432. [Google Scholar] [CrossRef]
- Segerer, S.; Banas, B.; Wornle, M.; Schmid, H.; Cohen, C.D.; Kretzler, M.; Mack, M.; Kiss, E.; Nelson, P.J.; Schlondorff, D.; et al. CXCR3 is involved in tubulointerstitial injury in human glomerulonephritis. Am. J. Pathol. 2004, 164, 635–649. [Google Scholar] [CrossRef]
- Lu, J.; Szeto, C.C.; Tam, L.S.; Lai, F.M.; Li, E.K.; Chow, K.M.; Li, P.K.; Kwan, B.C. Relationship of intrarenal gene expression and the histological class of lupus nephritis—A study on repeat renal biopsy. J. Rheumatol. 2012, 39, 1942–1947. [Google Scholar] [CrossRef] [PubMed]
- Avihingsanon, Y.; Phumesin, P.; Benjachat, T.; Akkasilpa, S.; Kittikowit, V.; Praditpornsilpa, K.; Wongpiyabavorn, J.; Eiam-Ong, S.; Hemachudha, T.; Tungsanga, K.; et al. Measurement of urinary chemokine and growth factor messenger RNAs: A noninvasive monitoring in lupus nephritis. Kidney Int. 2006, 69, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lit, L.C.W.; Wong, C.K.; Tam, L.S.; Li, E.K.M.; Lam, C.W.K. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2006, 65, 209–215. [Google Scholar] [CrossRef] [Green Version]
- Bauer, J.W.; Petri, M.; Batliwalla, F.M.; Koeuth, T.; Wilson, J.; Slattery, C.; Panoskaltsis-Mortari, A.; Gregersen, P.K.; Behrens, T.W.; Baechler, E.C. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: A validation study. Arthritis Rheum. 2009, 60, 3098–3107. [Google Scholar] [CrossRef] [Green Version]
- Kong, K.O.; Tan, A.W.; Thong, B.Y.H.; Lian, T.Y.; Cheng, Y.K.; Teh, C.L.; Koh, E.T.; Chng, H.H.; Law, W.G.; Lau, T.C.; et al. Enhanced expression of interferon-inducible protein-10 correlates with disease activity and clinical manifestations in systemic lupus erythematosus. Clin. Exp. Immunol. 2009, 156, 134–140. [Google Scholar] [CrossRef]
- Narumi, S.; Takeuchi, T.; Kobayashi, Y.; Konishi, K. Serum levels of ifn-inducible PROTEIN-10 relating to the activity of systemic lupus erythematosus. Cytokine 2000, 12, 1561–1565. [Google Scholar] [CrossRef]
- Rose, T.; Grutzkau, A.; Klotsche, J.; Enghard, P.; Flechsig, A.; Keller, J.; Riemekasten, G.; Radbruch, A.; Burmester, G.R.; Dorner, T.; et al. Are interferon-related biomarkers advantageous for monitoring disease activity in systemic lupus erythematosus? A longitudinal benchmark study. Rheumatol. (Oxf. Engl. ) 2017, 56, 1618–1626. [Google Scholar] [CrossRef] [Green Version]
- Watson, L.; Midgley, A.; Pilkington, C.; Tullus, K.; Marks, S.; Holt, R.; Jones, C.; Beresford, M. Urinary monocyte chemoattractant protein 1 and alpha 1 acid glycoprotein as biomarkers of renal disease activity in juvenile-onset systemic lupus erythematosus. Lupus 2012, 21, 496–501. [Google Scholar] [CrossRef]
- Björkander, S.; Bremme, K.; Persson, J.O.; van Vollenhoven, R.F.; Sverremark-Ekström, E.; Holmlund, U. Pregnancy-associated inflammatory markers are elevated in pregnant women with systemic lupus erythematosus. Cytokine 2012, 59, 392–399. [Google Scholar] [CrossRef]
- Eriksson, C.; Eneslätt, K.; Ivanoff, J.; Rantapää-Dahlqvist, S.; Sundqvist, K.G. Abnormal expression of chemokine receptors on T-cells from patients with systemic lupus erythematosus. Lupus 2003, 12, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Tokano, Y.; Nakano, S.; Watanabe, T.; Tamayama, Y.; Mitsuo, A.; Suzuki, J.; Kaneko, H.; Sekigawa, I.; Takasaki, Y. Chemoattractant mechanism of Th1 cells in class III and IV lupus nephritis. Autoimmunity 2009, 42, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.T.; Wong, C.K.; Tam, L.S.; Li, E.K.; Chen, D.P.; Lam, C.W. Decreased expression of T lymphocyte co-stimulatory molecule CD26 on invariant natural killer T cells in systemic lupus erythematosus. Immunol. Invest. 2009, 38, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Wanchu, A.; Bhatnagar, A. Interplay of cytokines and chemokines in the pathogenesis of Systemic Lupus Erythematosus. Am. J. Immunol. 2011, 7, 29–38. [Google Scholar]
- Abujam, B.; Cheekatla, S.S.; Aggarwal, A. Urinary CXCL-10/IP-10 and MCP-1 as markers to assess activity of lupus nephritis. Lupus 2013, 22, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Hrycek, E.; Franek, A.; Blaszczak, E.; Dworak, J.; Hrycek, A. Serum levels of selected chemokines in systemic lupus erythematosus patients. Rheumatol. Int. 2013, 33, 2423–2427. [Google Scholar] [CrossRef]
- Marie, M.A.; Abu Khalil, R.E.; Habib, H.M. Urinary CXCL10: A marker of nephritis in lupus patients. Reumatismo 2013, 65, 292–297. [Google Scholar] [CrossRef]
- Rose, T.; Grutzkau, A.; Hirseland, H.; Huscher, D.; Dahnrich, C.; Dzionek, A.; Ozimkowski, T.; Schlumberger, W.; Enghard, P.; Radbruch, A.; et al. IFNalpha and its response proteins, IP-10 and SIGLEC-1, are biomarkers of disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 2013, 72, 1639–1645. [Google Scholar] [CrossRef]
- Doe, K.; Nozawa, K.; Hiruma, K.; Yamada, Y.; Matsuki, Y.; Nakano, S.; Ogasawara, M.; Nakano, H.; Ikeda, T.; Ikegami, T.; et al. Antibody against chromatin assembly factor-1 is a novel autoantibody specifically recognized in systemic lupus erythematosus. Lupus 2014, 23, 1031–1041. [Google Scholar] [CrossRef]
- Dominguez-Gutierrez, P.R.; Ceribelli, A.; Satoh, M.; Sobel, E.S.; Reeves, W.H.; Chan, E.K. Elevated signal transducers and activators of transcription 1 correlates with increased C-C motif chemokine ligand 2 and C-X-C motif chemokine 10 levels in peripheral blood of patients with systemic lupus erythematosus. Arthritis Res. Ther. 2014, 16, R20. [Google Scholar] [CrossRef]
- El-Gohary, A.; Hegazy, A.; Abbas, M.; Kamel, N.; Nasef, S.I. Serum and Urinary Interferon-Gamma-Inducible Protein 10 in Lupus Nephritis. J. Clin. Lab. Anal. 2016, 30, 1135–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, J.Y.; Kim, S.K. Serum TWEAK as a biomarker for disease activity of systemic lupus erythematosus. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2016, 65, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Odler, B.; Bikov, A.; Streizig, J.; Balogh, C.; Kiss, E.; Vincze, K.; Barta, I.; Horvath, I.; Muller, V. CCL21 and IP-10 as blood biomarkers for pulmonary involvement in systemic lupus erythematosus patients. Lupus 2017, 26, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Oke, V.; Brauner, S.; Larsson, A.; Gustafsson, J.; Zickert, A.; Gunnarsson, I.; Svenungsson, E. IFN-λ1 with Th17 axis cytokines and IFN-α define different subsets in systemic lupus erythematosus (SLE). Arthritis Res. Ther. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Cai, B.; Zhang, J.; Shen, B.; Huang, Z.; Tan, C.; Baan, C.C.; Wang, L. IL-1 β and IL-6 Are Highly Expressed in RF+IgE+ Systemic Lupus Erythematous Subtype. J. Immunol. Res. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. Jasn 2004, 15, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Alharazy, S.; Kong, N.C.; Mohd, M.; Shah, S.A.; Ba’in, A.; Abdul Gafor, A.H. Urine Monocyte Chemoattractant Protein-1 and Lupus Nephritis Disease Activity: Preliminary Report of a Prospective Longitudinal Study. Autoimmune Dis. 2015, 2015, 962046. [Google Scholar] [CrossRef]
- Ronnblom, L.; Leonard, D. Interferon pathway in SLE: One key to unlocking the mystery of the disease. Lupus Sci. Med. 2019, 6, e000270. [Google Scholar] [CrossRef]
- Bai, Y.; Tong, Y.; Liu, Y.; Hu, H. Self-dsDNA in the pathogenesis of systemic lupus erythematosus. Clin. Exp. Immunol. 2018, 191, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reveille, J.D. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus 2004, 13, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Bax, L.; Yu, L.M.; Ikeda, N.; Moons, K.G. A systematic comparison of software dedicated to meta-analysis of causal studies. Bmc Med. Res. Methodol. 2007, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj (Clin. Res. Ed.) 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]


| Study | Country | N | SLE Patients | LN Patients | Disease Activity Criteria | Specimen | IP-10 Assay |
|---|---|---|---|---|---|---|---|
| Narumi (2000) [17] | Japan | 65 | 28 | 10 | NR | Serum | ELISA (in-house) |
| Eriksson (2003) [21] | Sweden | 45 | 23 | 10 | SLEDAI ≥ 6 | Serum | ELISA (R&D) |
| Avihingsanon (2006) [13] | Thailand | 36 | 26 | 26 | ISN/RPS class IV LN | Urine | qRT-PCR |
| Lit (2006) [14] | Hong Kong | 120 | 80 | 26 | SLEDAI ≥ 6 | Serum | CBA |
| Bauer (2009) [15] | US | 267 | 267 | NR | SLEDAI ≥ 6 | Serum | ELISA (SearchLight) |
| Kong (2009) [16] | Singapore | 514 | 464 | NR | SLAM-R > 5 * | Serum | ELISA (OptEIA) |
| Morimoto (2009) [22] | Japan | 71 | 41 | 41 | SLEDAI > 8 | Serum | ELISA (BD BioScience) |
| Wong (2009) [23] | Hong Kong | 37 | 23 | NR | NR | Serum | CBA |
| Shah (2011) [24] | India | 60 | 30 | NR | SLEDAI ** | Serum | ELISA (OptEIA) |
| Bjorkander (2012) [20] | Sweden | 35 | 15 | 3 | SLEDAI ≥ 4 | Serum | CBA |
| Watson (2012) [19] | UK | 83 | 60 | 8 | BILAG A or B | Urine | ELISA (R&D) |
| Abujam (2013) [25] | India | 136 | 136 | 46 | SELENA-SLEDAI ≥ 4, active LN if proteinuria ≥ 500 mg/day or active urinary sediment | Serum & urine | ELISA (OptEIA) |
| Hrycek (2013) [26] | Poland | 77 | 48 | NR | NR | Serum | ELISA (RAYBIO) |
| Marie (2013) [27] | Egypt | 60 | 60 | 30 | NR | Urine | ELISA (OptEIA) |
| Rose (2013) [28] | Germany | 106 | 79 | 17 | BILAG (not clearly stated) | Serum | ELISA (R&D) |
| Doe (2014) [29] | Japan | 189 | 80 | NR | NR | Serum | ELISA (R&D) |
| Dominguez-Gutierrez (2014) [30] | US | 168 | 103 | NR | SLEDAI > 4 | Serum | qRT-PCR |
| El-Gohary (2016) [31] | Egypt | 40 | 30 | 6 | SLEDAI ≥ 4, active LN if proteinuria ≥ 500 mg/day or active urinary sediment | Serum & urine | ELISA (R&D) |
| Choe (2016) [32] | Korea | 131 | 70 | 32 | SLEDAI ≥ 6 | Serum & urine | ELISA (R&D) |
| Odler (2017) [33] | Hungary | 27 | 18 | NR | NR | Serum | ELISA (custom made array kits) |
| Oke (2017) [34] | Sweden | 522 | 261 | NR | SLEDAI > 6 | Serum | ELISA (R&D) |
| Wu (2017) [35] | China | 191 | 111 | NR | NR | Serum | Multiplex immunoassay (Bio-Plex 200) |
| Rose (2017) [18] | Germany | 26 | 26 | NR | BILAG index classifying patients into flare, inactive, and remitting status | Serum | ELISA (R&D) |
| Study Outcomes | Number of Studies/Study Arms | Number of Patients | Mean Difference (95% CI) | p-Value | I2 Index a | p-Value | Egger′s Test b p-Value |
|---|---|---|---|---|---|---|---|
| Serum IP-10 in SLE (pg/mL) | |||||||
| • Serum IP-10 in SLE vs healthy controls | 8/8 | 1069 | 153.86 (91.63 to 216.10) | <0.001 | 87.1 | <0.001 | 0.04 |
| • Serum IP-10 in active SLE vs inactive SLE | 4/5 | 879 | 356.51 (59.57 to 653.44) | 0.019 | 95.9 | <0.001 | 0.28 |
| Serum IP-10 in LN (pg/mL) | |||||||
| • Serum IP-10 in LN vs healthy controls | 3/3 | 193 | 183.84 (126.54 to 241.14) | <0.001 | 0 | 0.37 | 0.49 |
| • Serum IP-10 in LN vs SLE without LN c | 5/7 | 402 | 22.62 (−182.83 to 228.08) | 0.83 | 86.9 | <0.001 | 0.22 |
| Urine IP-10 in SLE (pg/mgCr x 100) | |||||||
| • Urine IP-10 in SLE vs healthy controls | 2/2 | 95 | 0.21 (−0.74 to 1.15) | 0.67 | 54.3 | 0.14 | NA |
| • Urine IP-10 in SLE vs inactive SLE | 2/2 | 156 | 2.81 (−2.40 to 8.01) | 0.29 | 90.1 | 0.001 | NA |
| Urine IP-10 in LN (pg/mgCr x 100) | |||||||
| • Urine IP-10 in LN vs healthy controls | 2/2 | 51 | 0.21 (−0.92 to 1.33) | 0.72 | 17.2 | 0.27 | NA |
| • Urine IP-10 in LN vs SLE without LN c | 3/5 | 282 | 3.47 (−0.18 to 7.12) | 0.06 | 88.2 | <0.001 | 0.13 |
| Study Outcomes | Number of Studies/Study Arms | Number of Patients | Pooled Correlation Coefficient (95% CI) | p-Value | I2 index a | p-Value | Egger’s Test b p-Value |
|---|---|---|---|---|---|---|---|
| Serum IP-10 and disease activity | |||||||
| • Serum IP-10 and SLEDAI | 7/7 | 802 | 0.29 (0.22 to 0.35) | <0.001 | 0 | 0.61 | 0.48 |
| • Serum IP-10 and BILAG index | 2/2 | 105 | 0.41 (0.24 to 0.56) | <0.001 | 0 | 0.55 | NA |
| Urine IP-10 and disease activity | |||||||
| • Urine IP-10 and SLEDAI | 3/3 | 236 | 0.21 (0.05 to 0.36) | 0.011 | 27.4 | 0.25 | 0.53 |
| • Urine IP-10 and renal SLEDAI | 3/3 | 236 | 0.29 (0.05 to 0.50) | 0.019 | 73.8 | 0.02 | 0.85 |
| Serum IP-10 and other biomarkers | |||||||
| • Serum IP-10 and complement level | 3/5 | 1096 | −0.20 (−0.30 to −0.10) | <0.001 | 51.3 | 0.08 | 0.08 |
| • Serum IP-10 and anti-dsDNA and ESR | 3/5 | 1096 | 0.28 (0.15 to 0.40) | <0.001 | 70.3 | 0.01 | 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puapatanakul, P.; Chansritrakul, S.; Susantitaphong, P.; Ueaphongsukkit, T.; Eiam-Ong, S.; Praditpornsilpa, K.; Kittanamongkolchai, W.; Avihingsanon, Y. Interferon-Inducible Protein 10 and Disease Activity in Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4954. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194954
Puapatanakul P, Chansritrakul S, Susantitaphong P, Ueaphongsukkit T, Eiam-Ong S, Praditpornsilpa K, Kittanamongkolchai W, Avihingsanon Y. Interferon-Inducible Protein 10 and Disease Activity in Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2019; 20(19):4954. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194954
Chicago/Turabian StylePuapatanakul, Pongpratch, Sonchai Chansritrakul, Paweena Susantitaphong, Thornthun Ueaphongsukkit, Somchai Eiam-Ong, Kearkiat Praditpornsilpa, Wonngarm Kittanamongkolchai, and Yingyos Avihingsanon. 2019. "Interferon-Inducible Protein 10 and Disease Activity in Systemic Lupus Erythematosus and Lupus Nephritis: A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 20, no. 19: 4954. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20194954