The Influence of a Nanopatterned Scaffold that Mimics Abnormal Renal Mesangial Matrix on Mesangial Cell Behavior
Abstract
:1. Introduction
2. Results
2.1. Design and Fabrication of Disease- and Normal-mimic Nanopatterned TiO2 Substrates
2.2. MES13 Cell Proliferation on Disease-Mimic Nanopatterns
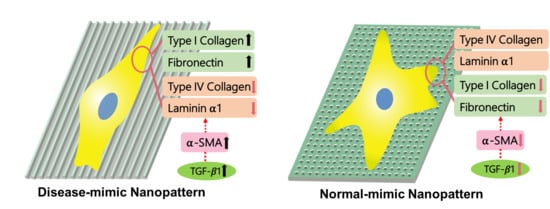
2.3. Expressions of Specific ECM Components in MES13 Cells
2.4. MES13 Cell Morphological and Cytoskeletal Changes
2.5. α-SMA Expression in MES13 Cells
2.6. TGF- β1 Expression in MES13 Cells
2.7. Integrin α5β1 Expression in MES13 Cells
3. Discussion
4. Materials and Methods
4.1. Fabrication of Nanopatterned TiO2 Substrates
4.2. Cell Culture
4.3. Cell Proliferation Assays
4.4. Immunofluorescence Staining and Confocal Imaging
4.5. SEM for Cell Morphology
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MM | Mesangial matrix |
| COL4 | Type IV collagen |
| COL1 | Type I collagen |
| MCs | Mesangial cells |
| α-SMA | Alpha-smooth muscle actin |
| TGF-β1 | Transforming growth factor β1 |
| ECM | Extracellular matrix |
| EBL | Electron beam lithography |
| TiO2 | Titanium dioxide |
| ALD | Atomic layer deposition |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
| MES13 | Immortalized mesangial cell line, SV40MES13 |
| NGAL | Neutrophil gelatinase-associated lipocalin |
References
- Scindia, Y.M.; Deshmukh, U.S.; Bagavant, H. Mesangial pathology in glomerular disease: Targets for therapeutic intervention. Adv. Drug Deliv. Rev. 2010, 62, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Wang, P.C.; Huang, T.C.; Taniguchi, A. Change in Renal Glomerular Collagens and Glomerular Filtration Barrier-Related Proteins in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Int. J. Mol. Sci. 2019, 20, 1458. [Google Scholar] [CrossRef] [PubMed]
- Zylka, A.; Dumnicka, P.; Kusnierz-Cabala, B.; Gala-Bladzinska, A.; Ceranowicz, P.; Kucharz, J.; Zabek-Adamska, A.; Maziarz, B.; Drozdz, R.; Kuzniewski, M. Markers of Glomerular and Tubular Damage in the Early Stage of Kidney Disease in Type 2 Diabetic Patients. Mediat. Inflamm. 2018, 2018, 7659243. [Google Scholar] [CrossRef] [PubMed]
- Sporek, M.; Dumnicka, P.; Gala-Bladzinska, A.; Ceranowicz, P.; Warzecha, Z.; Dembinski, A.; Stepien, E.; Walocha, J.; Drozdz, R.; Kuzniewski, M.; et al. Angiopoietin-2 Is an Early Indicator of Acute Pancreatic-Renal Syndrome in Patients with Acute Pancreatitis. Mediat. Inflamm. 2016, 2016, 5780903. [Google Scholar] [CrossRef] [PubMed]
- Sporek, M.; Gala-Bladzinska, A.; Dumnicka, P.; Mazur-Laskowska, M.; Kielczewski, S.; Walocha, J.; Ceranowicz, P.; Kuzniewski, M.; Mitus, J.; Kusnierz-Cabala, B. Urine NGAL is useful in the clinical evaluation of renal function in the early course of acute pancreatitis. Folia. Med. Cracov. 2016, 56, 13–25. [Google Scholar]
- Zylka, A.; Gala-Bladzinska, A.; Dumnicka, P.; Ceranowicz, P.; Kuzniewski, M.; Gil, K.; Olszanecki, R.; Kusnierz-Cabala, B. Is Urinary NGAL Determination Useful for Monitoring Kidney Function and Assessment of Cardiovascular Disease? A 12-Month Observation of Patients with Type 2 Diabetes. Dis. Markers 2016, 2016, 8489543. [Google Scholar] [CrossRef] [PubMed]
- Giehl, K.; Graness, A.; Goppelt-Struebe, M. The small GTPase Rac-1 is a regulator of mesangial cell morphology and thrombospondin-1 expression. Am. J. Physiol. Ren. Physiol 2008, 294, F407–F413. [Google Scholar] [CrossRef]
- Schlondorff, D.; Banas, B. The mesangial cell revisited: No cell is an island. J. Am. Soc. Nephrol. 2009, 20, 1179–1187. [Google Scholar] [CrossRef]
- Guo, L.; Luo, S.; Du, Z.; Zhou, M.; Li, P.; Fu, Y.; Sun, X.; Huang, Y.; Zhang, Z. Targeted delivery of celastrol to mesangial cells is effective against mesangioproliferative glomerulonephritis. Nat. Commun. 2017, 8, 878. [Google Scholar] [CrossRef]
- Davies, M. The mesangial cell: A tissue culture view. Kidney Int. 1994, 45, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Kashgarian, M.; Sterzel, R.B. The pathobiology of the mesangium. Kidney Int. 1992, 41, 524–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubus, I.; L’Azou, B.; Gordien, M.; Delmas, Y.; Labouyrie, J.P.; Bonnet, J.; Combe, C. Cytoskeletal reorganization by mycophenolic acid alters mesangial cell migration and contractility. Hypertension 2003, 42, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Schena, F.P.; Gesualdo, L.; Grandaliano, G.; Montinaro, V. Progression of renal damage in human glomerulonephritides: Is there sleight of hand in winning the game? Kidney Int. 1997, 52, 1439–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, H.; Sakai, T. Cell biology of mesangial cells: The third cell that maintains the glomerular capillary. Anat. Sci. Int. 2017, 92, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Mitarai, T.; Maruyama, N.; Nagasawa, R.; Yoshida, H.; Sakai, O. Mesangial cell behavior in a three-dimensional extracellular matrix. Kidney Int. 1991, 40, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Alexakis, C.; Maxwell, P.; Bou-Gharios, G. Organ-specific collagen expression: Implications for renal disease. Nephron Exp. Nephrol. 2006, 102, e71–e75. [Google Scholar] [CrossRef]
- Glick, A.D.; Jacobson, H.R.; Haralson, M.A. Mesangial deposition of type I collagen in human glomerulosclerosis. Hum. Pathol. 1992, 23, 1373–1379. [Google Scholar] [CrossRef]
- Ortega-Velazquez, R.; Gonzalez-Rubio, M.; Ruiz-Torres, M.P.; Diez-Marques, M.L.; Iglesias, M.C.; Rodriguez-Puyol, M.; Rodriguez-Puyol, D. Collagen I upregulates extracellular matrix gene expression and secretion of TGF-beta 1 by cultured human mesangial cells. Am. J. Physiol. Cell Physiol 2004, 286, C1335–C1343. [Google Scholar] [CrossRef]
- Ruef, C.; Kashgarian, M.; Coleman, D.L. Mesangial cell-matrix interactions. Effects on mesangial cell growth and cytokine secretion. Am. J. Pathol. 1992, 141, 429–439. [Google Scholar]
- Knupp, C.; Squire, J.M. Molecular packing in network-forming collagens. Adv. Protein Chem. 2005, 70, 375–403. [Google Scholar]
- Yang, Y.; Wang, K.; Gu, X.; Leong, K.W. Biophysical Regulation of Cell Behavior-Cross Talk between Substrate Stiffness and Nanotopography. Eng. (Beijing) 2017, 3, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wei, H.; Han, M.; Guan, Y.; Wu, Y.; Li, H. Collagen type III glomerulopathy: A case report and review of 20 cases. Exp. Med. 2015, 10, 1445–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hironaka, K.; Makino, H.; Yamasaki, Y.; Ota, Z. Renal basement membranes by ultrahigh resolution scanning electron microscopy. Kidney Int. 1993, 43, 334–345. [Google Scholar] [CrossRef] [Green Version]
- Erisken, C.; Zhang, X.; Moffat, K.L.; Levine, W.N.; Lu, H.H. Scaffold fiber diameter regulates human tendon fibroblast growth and differentiation. Tissue Eng. Part. A 2013, 19, 519–528. [Google Scholar] [CrossRef]
- Bae, H.; Chu, H.; Edalat, F.; Cha, J.M.; Sant, S.; Kashyap, A.; Ahari, A.F.; Kwon, C.H.; Nichol, J.W.; Manoucheri, S.; et al. Development of functional biomaterials with micro- and nanoscale technologies for tissue engineering and drug delivery applications. J. Tissue Eng. Regen Med. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Ermis, M.; Antmen, E.; Hasirci, V. Micro and Nanofabrication methods to control cell-substrate interactions and cell behavior: A review from the tissue engineering perspective. Bioact Mater. 2018, 3, 355–369. [Google Scholar] [CrossRef]
- von der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale engineering of biomimetic surfaces: Cues from the extracellular matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef]
- Ozguldez, H.O.; Cha, J.; Hong, Y.; Koh, I.; Kim, P. Nanoengineered, cell-derived extracellular matrix influences ECM-related gene expression of mesenchymal stem cells. Biomater Res. 2018, 22, 32. [Google Scholar] [CrossRef]
- Abdellatef, S.A.; Ohi, A.; Nabatame, T.; Taniguchi, A. Induction of hepatocyte functional protein expression by submicron/nano-patterning substrates to mimic in vivo structures. Biomater. Sci. 2014, 2, 330–338. [Google Scholar] [CrossRef]
- Kim, D.; Li, H.Y.; Lee, J.H.; Oh, Y.S.; Jun, H.S. Lysophosphatidic acid increases mesangial cell proliferation in models of diabetic nephropathy via Rac1/MAPK/KLF5 signaling. Exp. Mol. Med. 2019, 51, 18. [Google Scholar] [CrossRef] [PubMed]
- Ishimura, E.; Sterzel, R.B.; Morii, H.; Kashgarian, M. Extracellular matrix protein: Gene expression and synthesis in cultured rat mesangial cells. Nihon Jinzo Gakkai Shi 1992, 34, 9–17. [Google Scholar] [PubMed]
- Bussolati, B.; Mariano, F.; Biancone, L.; Foa, R.; David, S.; Cambi, V.; Camussi, G. Interleukin-12 is synthesized by mesangial cells and stimulates platelet-activating factor synthesis, cytoskeletal reorganization, and cell shape change. Am. J. Pathol. 1999, 154, 623–632. [Google Scholar] [CrossRef]
- Thwaites, T.R.; Pedrosa, A.T.; Peacock, T.P.; Carabeo, R.A. Vinculin Interacts with the Chlamydia Effector TarP Via a Tripartite Vinculin Binding Domain to Mediate Actin Recruitment and Assembly at the Plasma Membrane. Front. Cell Infect. Microbiol. 2015, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Xia, W.; Fei, X.; Xu, Q.; Yang, X.; Qiu, D.; Wang, M. Relaxin Inhibits High Glucose-Induced Matrix Accumulation in Human Mesangial Cells by Interfering with TGF-beta1 Production and Mesangial Cells Phenotypic Transition. Biol. Pharm. Bull. 2015, 38, 1464–1469. [Google Scholar] [CrossRef]
- Iwano, M.; Akai, Y.; Fujii, Y.; Dohi, Y.; Matsumura, N.; Dohi, K. Intraglomerular expression of transforming growth factor-beta 1 (TGF-beta 1) mRNA in patients with glomerulonephritis: Quantitative analysis by competitive polymerase chain reaction. Clin. Exp. Immunol. 1994, 97, 309–314. [Google Scholar] [CrossRef]
- O’Connor, J.W.; Mistry, K.; Detweiler, D.; Wang, C.; Gomez, E.W. Cell-cell contact and matrix adhesion promote alphaSMA expression during TGFbeta1-induced epithelial-myofibroblast transition via Notch and MRTF-A. Sci. Rep. 2016, 6, 26226. [Google Scholar] [CrossRef]
- Stephenson, L.A.; Haney, L.B.; Hussaini, I.M.; Karns, L.R.; Glass, W.F., 2nd. Regulation of smooth muscle alpha-actin expression and hypertrophy in cultured mesangial cells. Kidney Int. 1998, 54, 1175–1187. [Google Scholar] [CrossRef]
- Adler, S.; Brady, H.R. Cell adhesion molecules and the glomerulopathies. Am. J. Med. 1999, 107, 371–386. [Google Scholar] [CrossRef]
- Canty, E.G.; Starborg, T.; Lu, Y.; Humphries, S.M.; Holmes, D.F.; Meadows, R.S.; Huffman, A.; O’Toole, E.T.; Kadler, K.E. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J. Biol. Chem. 2006, 281, 38592–38598. [Google Scholar] [CrossRef]
- Makino, H.; Shikata, K.; Hironaka, K.; Kushiro, M.; Yamasaki, Y.; Sugimoto, H.; Ota, Z.; Araki, N.; Horiuchi, S. Ultrastructure of nonenzymatically glycated mesangial matrix in diabetic nephropathy. Kidney Int. 1995, 48, 517–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, G.A.; Turbat-Herrera, E.A. Renal diseases with organized deposits: An algorithmic approach to classification and clinicopathologic diagnosis. Arch. Pathol Lab. Med. 2010, 134, 512–531. [Google Scholar] [PubMed]
- Cove-Smith, A.; Hendry, B.M. The regulation of mesangial cell proliferation. Nephron Exp. Nephrol. 2008, 108, e74–e79. [Google Scholar] [CrossRef] [PubMed]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef]
- Hubchak, S.C.; Sparks, E.E.; Hayashida, T.; Schnaper, H.W. Rac1 promotes TGF-beta-stimulated mesangial cell type I collagen expression through a PI3K/Akt-dependent mechanism. Am. J. Physiol Ren. Physiol. 2009, 297, F1316–F1323. [Google Scholar] [CrossRef]
- Brodeur, A.C.; Roberts-Pilgrim, A.M.; Thompson, K.L.; Franklin, C.L.; Phillips, C.L. Transforming growth factor-beta1/Smad3-independent epithelial-mesenchymal transition in type I collagen glomerulopathy. Int J. Nephrol Renov. Dis. 2017, 10, 251–259. [Google Scholar] [CrossRef]
- Ning, L.; Kurihara, H.; de Vega, S.; Ichikawa-Tomikawa, N.; Xu, Z.; Nonaka, R.; Kazuno, S.; Yamada, Y.; Miner, J.H.; Arikawa-Hirasawa, E. Laminin alpha1 regulates age-related mesangial cell proliferation and mesangial matrix accumulation through the TGF-beta pathway. Am. J. Pathol. 2014, 184, 1683–1694. [Google Scholar] [CrossRef]
- Hu, C.; Sun, L.; Xiao, L.; Han, Y.; Fu, X.; Xiong, X.; Xu, X.; Liu, Y.; Yang, S.; Liu, F.; et al. Insights into the Mechanisms Involved in the Expression and Regulation of Extracellular Matrix Proteins in Diabetic Nephropathy. Curr. Med. Chem. 2015, 22, 2858–2870. [Google Scholar] [CrossRef] [Green Version]
- Cortes, P.; Mendez, M.; Riser, B.L.; Guerin, C.J.; Rodriguez-Barbero, A.; Hassett, C.; Yee, J. F-actin fiber distribution in glomerular cells: Structural and functional implications. Kidney Int. 2000, 58, 2452–2461. [Google Scholar] [CrossRef] [Green Version]
- Lutz, R.; Pataky, K.; Gadhari, N.; Marelli, M.; Brugger, J.; Chiquet, M. Nano-stenciled RGD-gold patterns that inhibit focal contact maturation induce lamellipodia formation in fibroblasts. PLoS ONE 2011, 6, e25459. [Google Scholar] [CrossRef]
- Wang, K.; Bruce, A.; Mezan, R.; Kadiyala, A.; Wang, L.; Dawson, J.; Rojanasakul, Y.; Yang, Y. Nanotopographical Modulation of Cell Function through Nuclear Deformation. Acs Appl Mater. Interfaces 2016, 8, 5082–5092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdellatef, S.A.; Tange, R.; Sato, T.; Ohi, A.; Nabatame, T.; Taniguchi, A. Nanostructures Control the Hepatocellular Responses to a Cytotoxic Agent “Cisplatin”. Biomed. Res. Int 2015, 2015, 925319. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, T.D.; Holt, S.G.; Tan, S.J.; Wigg, B.; Samuel, C.S.; Smith, E.R. Epigenetic Modifications to H3K9 in Renal Tubulointerstitial Cells after Unilateral Ureteric Obstruction and TGF-beta1 Stimulation. Front. Pharm. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 2011, 92, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Brigstock, D.R. Integrin expression and function in the response of primary culture hepatic stellate cells to connective tissue growth factor (CCN2). J. Cell Mol. Med. 2011, 15, 1087–1095. [Google Scholar] [CrossRef]
- Weston, B.S.; Wahab, N.A.; Mason, R.M. CTGF mediates TGF-beta-induced fibronectin matrix deposition by upregulating active alpha5beta1 integrin in human mesangial cells. J. Am. Soc. Nephrol 2003, 14, 601–610. [Google Scholar] [CrossRef]
- Ghayur, M.N.; Krepinsky, J.C.; Janssen, L.J. Contractility of the Renal Glomerulus and Mesangial Cells: Lingering Doubts and Strategies for the Future. Med. Hypotheses Res. 2008, 4, 1–9. [Google Scholar]
- Oikonomou, K.; Kapsoritakis, A.; Eleftheriadis, T.; Stefanidis, I.; Potamianos, S. Renal manifestations and complications of inflammatory bowel disease. Inflamm. Bowel Dis. 2011, 17, 1034–1045. [Google Scholar] [CrossRef]
- Abdellatef, S.A.; Ohi, A.; Nabatame, T.; Taniguchi, A. The Effect of Physical and Chemical Cues on Hepatocellular Function and Morphology. Int. J. Mol. Sci. 2014, 15, 4299–4317. [Google Scholar] [CrossRef] [Green Version]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-J.; Minei, R.; Sato, T.; Taniguchi, A. The Influence of a Nanopatterned Scaffold that Mimics Abnormal Renal Mesangial Matrix on Mesangial Cell Behavior. Int. J. Mol. Sci. 2019, 20, 5349. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215349
Chang C-J, Minei R, Sato T, Taniguchi A. The Influence of a Nanopatterned Scaffold that Mimics Abnormal Renal Mesangial Matrix on Mesangial Cell Behavior. International Journal of Molecular Sciences. 2019; 20(21):5349. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215349
Chicago/Turabian StyleChang, Chia-Jung, Rin Minei, Takeshi Sato, and Akiyoshi Taniguchi. 2019. "The Influence of a Nanopatterned Scaffold that Mimics Abnormal Renal Mesangial Matrix on Mesangial Cell Behavior" International Journal of Molecular Sciences 20, no. 21: 5349. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215349