Neutral Sphingomyelinase Modulation in the Protective/Preventive Role of rMnSOD from Radiation-Induced Damage in the Brain
Abstract
:1. Introduction
2. Results
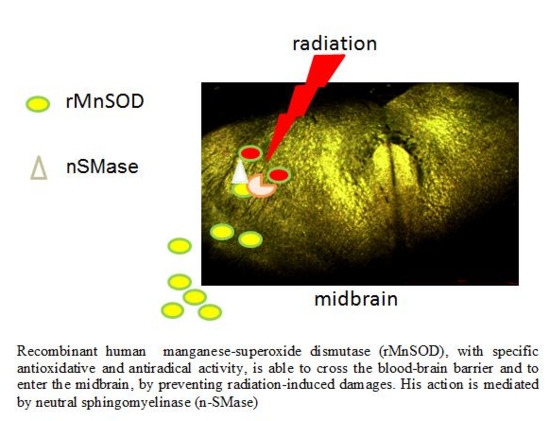
2.1. Protective and Preventive Effect of rMnSOD on Radiation-Induced Structural Changes in Midbrain Tissue
2.2. nSMase Is Required for the Protective and Preventive Effect of rMnSOD
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Design and Animal Care
4.3. Morphological and Immunohistochemistry Analysis
4.4. Reverse Transcription Quantitative PCR (RTqPCR)
4.5. Protein Concentration and Western Blotting
4.6. nSMaseActivity Assay
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Haimovitz-Friedman, A.; Kan, C.C.; Ehleiter, D.; Persaud, R.S.; McLoughlin, M.; Fuks, Z.; Kolesnick, R.N. Ionizingradiationacts on cellular membranes to generate ceramide and initiate apoptosis. J. Exp. Med. 1994, 180, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Chmura, S.J.; Mauceri, H.J.; Advani, S.; Heimann, R.; Beckett, M.A.; Nodzenski, E.; Quintans, J.; Kufe, D.W.; Weichselbaum, R.R. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinaseactivation increases tumor killing by ionizingradiation. Cancer Res. 1997, 57, 4340–4347. [Google Scholar] [PubMed]
- Albi, E.; Cataldi, S.; Lazzarini, A.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Radiation and thyroid cancer. Int. J. Mol. Sci. 2017, 18, E911. [Google Scholar] [CrossRef] [PubMed]
- Won, J.S.; Singh, I. Sphingolipid signaling and redox regulation. Free Radic Biol. Med. 2016, 40, 1875–1888. [Google Scholar]
- Kitatani, K.; Akiba, S.; Sato, T. Ceramide-induced enhancement of secretory phospholipase A2 expression via generation of reactive oxygen species in tumor necrosis factor-alpha-stimulated mesangial cells. Cell Signal. 2004, 16, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Junk, P.; Huwiler, A.; Burkhardt, C.; Wallerath, T.; Pfeilschifter, J.; Förstermann, U. Dual effect of ceramide on human endothelial cells: Induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation 2002, 106, 2250–2256. [Google Scholar] [CrossRef]
- Li, X.; Becker, K.A.; Zhang, Y. Ceramide in redox signaling and cardiovascular diseases. Cell. Physiol. Biochem. 2010, 26, 41–48. [Google Scholar] [CrossRef]
- Frazziano, G.; Moreno, L.; Moral-Sanz, J.; Menendez, C.; Escolano, L.; Gonzalez, C.; Villamor, E.; Alvarez-Sala, J.L.; Cogolludo, A.L.; Perez-Vizcaino, F. Neutral sphingomyelinase, NADPH oxidase and reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. J. Cell. Physiol. 2011, 226, 2633–2640. [Google Scholar] [CrossRef]
- JazvinšćakJembrek, M.; Hof, P.R.; Šimić, G. Ceramides in Alzheimer’s disease: Key mediators of neuronal apoptosis induced by oxidative stress and Aβ accumulation. Oxid. Med. Cell. Longev. 2015, 346783. [Google Scholar]
- Chen, Y.Y.; Hsu, M.J.; Sheu, J.R.; Lee, L.W.; Hsieh, C.Y. Andrographolide, a Novel NF-κB Inhibitor, Induces Vascular Smooth Muscle Cell Apoptosis via a Ceramide-p47phox-ROSSignaling Cascade. Evid. Based. Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Pahan, K.; Dobashi, K.; Ghosh, B.; Singh, I. Induction of the manganese superoxide dismutase gene by sphingomyelinase and ceramide. J. Neurochem. 1999, 73, 513–520. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Schuchman, E.H. Ceramide and ischemia/reperfusion injury. J. Lipids. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.A.; Zhang, Y.; Li, X.; Gulbins, E. Ceramide: A novel player in reactive oxygen species-induced signaling? Antioxid. Redox. Signal. 2007, 9, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Bezombes, C.; de Thonel, A.; Apostolou, A.; Louat, T.; Jaffrézou, J.P.; Laurent, G.; Quillet-Mary, A. Overexpression of protein kinase Czeta confers protection against antileukemic drugs by inhibiting the redox-dependentsphingomyelinaseactivation. Mol. Pharmacol. 2002, 62, 1446–1455. [Google Scholar] [CrossRef]
- Borrelli, A.; Schiattarella, A.; Mancini, R.; Morrica, B.; Cerciello, V.; Mormile, M.; D’Alesio, V.; Bottalico, L.; Morelli, F.; D’Armiento, M.; et al. A recombinant MnSOD is radioprotective for normal cells and radiosensitizing for tumor cells. Free Radic. Biol. Med. 2009, 46, 110–116. [Google Scholar] [CrossRef]
- Epperly, M.W.; Gretton, J.E.; Sikora, C.A.; Jefferson, M.; Bernarding, M.; Nie, S.; Greenberger, J.S. Mitochondrial localization of superoxide dismutase is required for decreasing radiation cellular damage. Radiat. Res. 2003, 160, 568–578. [Google Scholar] [CrossRef]
- Borrelli, A.; Schiattarella, A.; Bonelli, P.; Tuccillo, F.M.; Buonaguro, F.M.; Mancini, A. The functional role of MnSOD as a biomarker of human diseases and therapeutic potential of a new isoform of a human recombinant MnSOD. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Pica, A.; Di Santi, A.; D’Angelo, V.; Iannotta, A.; Ramaglia, M.; Di Martino, M.; Pollio, M.L.; Schiattarella, A.; Borrelli, A.; Mancini, A.; et al. Effect ofrMnSODon survival signaling in pediatric high risk T-cell acute lymphoblastic leukaemia. J. Cell Physiol. 2015, 230, 1086–1093. [Google Scholar] [CrossRef]
- Cataldi, S.; Codini, M.; Hunot, S.; Légeron, F.P.; Ferri, I.; Siccu, P.; Sidoni, A.; Ambesi-Impiombato, F.S.; Beccari, T.; Curcio, F.; et al. e-Cadherin in 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Parkinson Disease. Mediators Inflamm. 2016, 2016. [Google Scholar] [CrossRef]
- Albi, E.; Ambesi-Impiombato, S.; Villani, M.; DePol, I.; Spelat, R.; Lazzarini, R.; Perrella, G. Thyroid cell growth: Sphingomyelin metabolism as non-invasive marker for cell damage acquired during spaceflight. Astrobiology 2018, 10, 811–820. [Google Scholar] [CrossRef]
- Le, O.; Palacio, L.; Bernier, G.; Batinic-Haberle, I.; Hickson, G.; Beauséjour, C. INK4a/ARF Expression Impairs Neurogenesis in theBrainof Irradiated Mice. Stem. Cell Rep. 2018, 10, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Constanzo, J.; Dumont, M.; Lebel, R.; Tremblay, L.; Whittingstall, K.; Masson-Côté, L.; Geha, S.; Sarret, P.; Lepage, M.; Paquette, B.; et al. Diffusion MRI monitoring of specific structures in the irradiated ratbrain. Magn. Reson. Med. 2018, 80, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Ferri, I.; Sidoni, A.; Traina, G.; Fettucciari, K.; Ambesi-Impiombato, F.S.; Lazzarini, A.; Curcio, F.; Ceccarini, M.R.; et al. VDR independent induction of acid-sphingomyelinase by 1,23(OH)2 D3 in gastric cancer cells: Impact on apoptosis and cell morphology. Biochimie 2018, 146, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Ceccarini, M.R.; Codini, M.; Cataldi, S.; Vannini, S.; Lazzarini, A.; Floridi, A.; Moretti, M.; Villarini, M.; Fioretti, B.; Beccari, T.; et al. Acid sphingomyelinaseas target of LyciumChinense: Promising new action for cell health. Lipids. Health. Dis. 2016, 15, 183. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldi, S.; Borrelli, A.; Ceccarini, M.R.; Nakashidze, I.; Codini, M.; Belov, O.; Ivanov, A.; Krasavin, E.; Ferri, I.; Conte, C.; et al. Neutral Sphingomyelinase Modulation in the Protective/Preventive Role of rMnSOD from Radiation-Induced Damage in the Brain. Int. J. Mol. Sci. 2019, 20, 5431. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215431
Cataldi S, Borrelli A, Ceccarini MR, Nakashidze I, Codini M, Belov O, Ivanov A, Krasavin E, Ferri I, Conte C, et al. Neutral Sphingomyelinase Modulation in the Protective/Preventive Role of rMnSOD from Radiation-Induced Damage in the Brain. International Journal of Molecular Sciences. 2019; 20(21):5431. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215431
Chicago/Turabian StyleCataldi, Samuela, Antonella Borrelli, Maria Rachele Ceccarini, Irina Nakashidze, Michela Codini, Oleg Belov, Alexander Ivanov, Eugene Krasavin, Ivana Ferri, Carmela Conte, and et al. 2019. "Neutral Sphingomyelinase Modulation in the Protective/Preventive Role of rMnSOD from Radiation-Induced Damage in the Brain" International Journal of Molecular Sciences 20, no. 21: 5431. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215431