Clinical Response to Personalized Exercise Therapy in Heart Failure Patients with Reduced Ejection Fraction Is Accompanied by Skeletal Muscle Histological Alterations
Abstract
:1. Introduction
2. Results
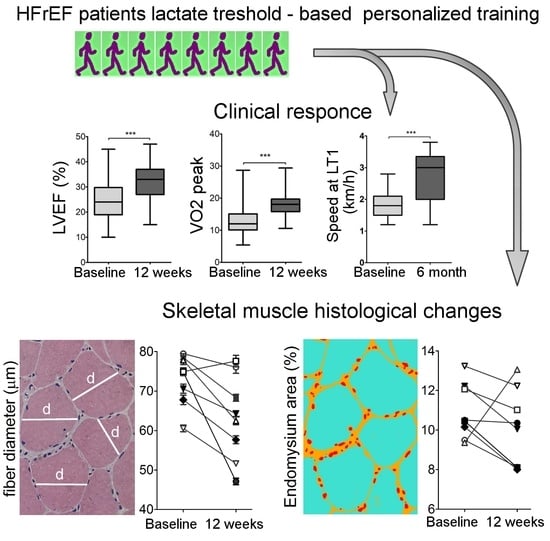
2.1. Description of Patients’ Cohort and the Effects of Personalized Training on Clinical Parameters
2.2. Physiological Response To Exercise
2.3. Muscle Histology
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Cardiopulmonary Exercise Testing and Lactate Threshold Determination
4.3. Echocardiography
4.4. Quality of Life and Exercise Tolerance Tests
4.5. Exercise Therapy Protocol
4.6. Histology
4.7. Determination of Endomysium Area
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zizola, C.; Schulze, P.C. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail. Rev. 2013, 18, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Ritterhoff, J.; Tian, R. Metabolismin cardiomyopathy: Every substrate matters. Cardiovasc. Res. 2017, 113, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Gosker, H.R.; Wouters, E.F.; van der Vusse, G.J.; Schols, A.M. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: Underlying mechanisms and therapy perspectives. Am. J. Clin. Nutr. 2000, 71, 1033–1047. [Google Scholar] [CrossRef] [PubMed]
- Springer, J.; Springer, J.-I.; Anker, S.D. Muscle wasting and sarcopenia in heart failure and beyond: Update 2017. ESC Hear. Fail. 2017, 4, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Loncar, G.; Springer, J.; Anker, M.; Doehner, W.; Lainscak, M. Cardiac cachexia: Hic et nunc. J. Cachexia. Sarcopenia Muscle 2016, 7, 246–260. [Google Scholar] [CrossRef]
- Vescovo, G. Skeletal muscle response to exercise and treatment: Another sibyl in the heart failure syndrome? Int. J. Cardiol. 2002, 83, 33–34. [Google Scholar] [CrossRef]
- Dmitrieva, R.I.; Revittser, A.V.; Klukina, M.A.; Sviryaev, Y.V.; Korostovtseva, L.S.; Kostareva, A.A.; Zaritskey, A.Y.; Shlyakhto, E.V. Functional properties of bone marrow derived multipotent mesenchymal stromal cells are altered in heart failure patients, and could be corrected by adjustment of expansion strategies. Aging (Albany. NY) 2015, 7, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Dmitrieva, R.I.; Lelyavina, T.A.; Komarova, M.Y.; Galenko, V.L.; Ivanova, O.A.; Tikanova, P.A.; Khromova, N.V.; Golovkin, A.S.; Bortsova, M.A.; Sergushichev, A.; et al. Skeletal muscle resident progenitor cells coexpress mesenchymal and myogenic markers and are not affected by chronic heart failure-induced dysregulations. Stem Cells Int. 2019, 2019, 1–11. [Google Scholar] [CrossRef]
- Tomono, J.; Adachi, H.; Oshima, S.; Kurabayashi, M. Usefulness of anaerobic threshold to peak oxygen uptake ratio to determine the severity and pathophysiological condition of chronic heart failure. J. Cardiol. 2016, 68, 373–378. [Google Scholar] [CrossRef]
- Gitt, A.K.; Wasserman, K.; Kilkowski, C.; Kleemann, T.; Kilkowski, A.; Bangert, M.; Schneider, S.; Schwarz, A.; Senges, J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002, 106, 3079–3084. [Google Scholar] [CrossRef]
- Carriere, C.; Corrà, U.; Piepoli, M.; Bonomi, A.; Merlo, M.; Barbieri, S.; Salvioni, E.; Binno, S.; Mapelli, M.; Righini, F.; et al. Anaerobic Threshold and Respiratory Compensation Point Identification During Cardiopulmonary Exercise Tests in Chronic Heart Failure. Chest 2019, 156, 338–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Dumitrescu, D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int. J. Cardiol. 2019, 288, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Agostoni, P.; Arena, R.; Belardinelli, R.; Dumitrescu, D.; Hager, A.; Myers, J.; Rauramaa, R.; Riley, M.; Takken, T.; et al. The Role of Gas Exchange Variables in Cardiopulmonary Exercise Testing for Risk Stratification and Management of Heart Failure with Reduced Ejection Fraction. Am. Heart J. 2018, 202, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Lelyavina, T.; Sitnikova, M. Shlyakhto Eugeny Diagnostic and prognostic value of lactate threshold and pH - threshold determination during cardiopulmonary testing in patients with chronic heart failure. Br. J. Med. Med. Res. 2015, 5, 289–296. [Google Scholar] [CrossRef]
- Lelyavina, T.; Sitnikova, M.; Galenko, V.; Bortzova, M. Aerobic training in heart failure patients with optimal heart failure therapy—A prospective randomized STUDY. World J. Pharm. Res. 2017, 6, 59–67. [Google Scholar]
- Galenko, V.L.; Lelyavina, T.A.; Sitnikova, M.Y. Response predictors for physical training HFrEF patients. Kardiologiia 2018, S4, 22–28. [Google Scholar] [CrossRef]
- Lelyavina, A.T.; Sitnikova, M.Y.; Beresina, A.V.; Kozlenok, A.V.; Shlyakhto, E.V. New Approaches to Marking Stages of Incremental Physical Work by Example of Cardiopulmonary Exercise Testing. J. US China Med. Sci. 2016, 11, 9–13. [Google Scholar]
- Binder, R.K.; Wonisch, M.; Corra, U.; Cohen-Solal, A.; Vanhees, L.; Saner, H.; Schmid, J.P. Methodological approach to the first and second lactate threshold in incremental cardiopulmonary exercise testing. Eur. J. Prev. Cardiol. 2008, 15, 726–734. [Google Scholar] [CrossRef]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef]
- Lunde, P.K.; Sjaastad, I.; Schiøtz Thorud, H.M.; Sejersted, O.M. Skeletal muscle disorders in heart failure. Acta Physiol. Scand. 2001, 171, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Kemp, G.J.; Thompson, C.H.; Stratton, J.R.; Brunotte, F.; Conway, M.; Adamopoulos, S.; Arnolda, L.; Radda, G.K.; Rajagopalan, B. Abnormalities in exercising skeletal muscle in congestive heart failure can be explained in terms of decreased mitochondrial ATP synthesis, reduced metabolic efficiency, and increased glycogenolysis. Heart 1996, 76, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Lavine, K.J.; Sierra, O.L. Skeletal muscle inflammation and atrophy in heart failure. Heart Fail. Rev. 2017, 22, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ades, P.A.; Waldmann, M.L.; Meyer, W.L.; Brown, K.A.; Poehlman, E.T.; Pendlebury, W.W.; Leslie, K.O.; Gray, P.R.; Lew, R.R.; LeWinter, M.M. Skeletal muscle and cardiovascular adaptations to exercise conditioning in older coronary patients. Circulation 1996, 94, 323–330. [Google Scholar] [CrossRef]
- Larsen, A.I.; Lindal, S.; Aukrust, P.; Toft, I.; Aarsland, T.; Dickstein, K. Effect of exercise training on skeletal muscle fibre characteristics in men with chronic heart failure. Correlation between skeletal muscle alterations, cytokines and exercise capacity. Int. J. Cardiol. 2002, 83, 25–32. [Google Scholar] [CrossRef]
- Stecco, A.; Stern, R.; Fantoni, I.; De Caro, R.; Stecco, C. Fascial Disorders: Implications for Treatment. PM R 2016, 8, 161–168. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Józsa, L.; Kannus, P.; Järvinen, T.L.N.; Järvinen, M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. J. Muscle Res. Cell Motil. 2002, 23, 245–254. [Google Scholar] [CrossRef]
- Takala, T.E.; Virtanen, P. Biochemical composition of muscle extracellular matrix: The effect of loading. Scand. J. Med. Sci. Sport. 2000, 10, 321–325. [Google Scholar] [CrossRef]
- Beckers, P.J.; Possemiers, N.M.; Van Craenenbroeck, E.M.; Van Berendoncks, A.M.; Wuyts, K.; Vrints, C.J.; Conraads, V.M. Comparison of three methods to identify the anaerobic threshold during maximal exercise testing in patients with chronic heart failure. Am. J. Phys. Med. Rehabil. 2012, 91, 148–155. [Google Scholar] [CrossRef]
- De Bruin, M.; Smeulders, M.J.; Kreulen, M.; Huijing, P.A.; Jaspers, R.T. Intramuscular Connective Tissue Differences in Spastic and Control Muscle: A Mechanical and Histological Study. PLoS ONE 2014, 9, 101038. [Google Scholar] [CrossRef]




| Age, years | 53 + 4 |
| Male/female, n | 107/37 |
| BMI, kg/m2 | 26.6 + 2.5 |
| NYHA III, n | 144 |
| LVEF, % | 26 + 7 |
| VO2 peak (ml/kg/min) | 12.9 + 3.8 |
| Disease etiology (DCM/CAD), n (%) | 48/96 (67/33) |
| ACEI/Beta-blockers/Diuretics (%) | 100/100/100 |
| Cardiac resynchronization therapy, n (%) | 31 (22) |
| Coronary artery bypass graft, n (%) | 43 (30) |
| COPD, n (%) | 52 (36) |
| Atrial fibrillation, n (%) | 18 (12) |
| Anemia, n (%) | 7 (5) |
| Donors | HF1 | HF2 | HF3 | HF4 | HF5 | HF6 | HF7 | HF8 |
| Age, years | 56 | 48 | 63 | 61 | 56 | 62 | 52 | 54 |
| BMI, kg/m2 | 27.07 | 26.46 | 24,1 | 32.87 | 26.77 | 23.32 | 29.5 | 26.12 |
| LVEF, % | 25 | 20 | 11 | 24 | 28 | 40 | 15 | 30 |
| VO2 peak (ml/kg/min) | 16.2 | 13.6 | 17.3 | 11 | 13.1 | 22.5 | 14.7 | 28.2 |
| Disease etiology (DCM/ICM) | DCM | DCM | CAD | CAD | CAD | CAD | CAD | DCM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lelyavina, T.A.; Galenko, V.L.; Ivanova, O.A.; Komarova, M.Y.; Ignatieva, E.V.; Bortsova, M.A.; Yukina, G.Y.; Khromova, N.V.; Sitnikova, M.Y.; Kostareva, A.A.; et al. Clinical Response to Personalized Exercise Therapy in Heart Failure Patients with Reduced Ejection Fraction Is Accompanied by Skeletal Muscle Histological Alterations. Int. J. Mol. Sci. 2019, 20, 5514. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215514
Lelyavina TA, Galenko VL, Ivanova OA, Komarova MY, Ignatieva EV, Bortsova MA, Yukina GY, Khromova NV, Sitnikova MY, Kostareva AA, et al. Clinical Response to Personalized Exercise Therapy in Heart Failure Patients with Reduced Ejection Fraction Is Accompanied by Skeletal Muscle Histological Alterations. International Journal of Molecular Sciences. 2019; 20(21):5514. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215514
Chicago/Turabian StyleLelyavina, Tatiana A., Victoria L. Galenko, Oksana A. Ivanova, Margarita Y. Komarova, Elena V. Ignatieva, Maria A. Bortsova, Galina Y. Yukina, Natalia V. Khromova, Maria Yu. Sitnikova, Anna A. Kostareva, and et al. 2019. "Clinical Response to Personalized Exercise Therapy in Heart Failure Patients with Reduced Ejection Fraction Is Accompanied by Skeletal Muscle Histological Alterations" International Journal of Molecular Sciences 20, no. 21: 5514. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20215514