Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma
Abstract
:1. Introduction
2. Results
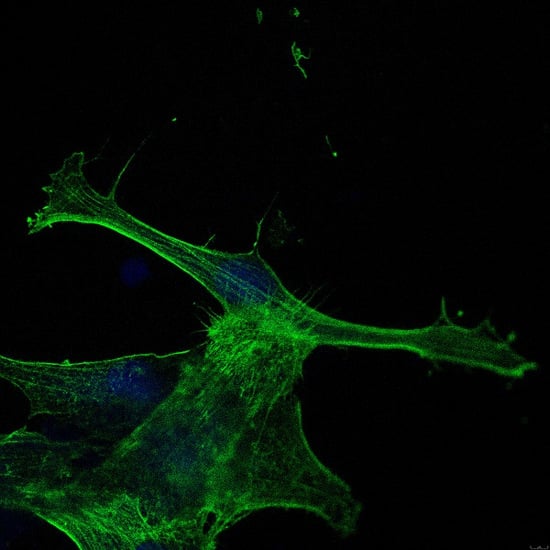
2.1. Cell Attachment and Morphology
2.2. Viability
2.3. Toxicity
3. Discussion
4. Materials and Methods
4.1. Sample Preparation and Surface Characterization
4.2. UV Light and NTP Treatment
4.3. Murine Fibroblast L929 and HGF Cell Culture
4.4. Cell Attachment and Morphology
4.5. Viability Assay
4.6. Cytotoxicity Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HGFs | Human gingival fibroblast |
| NTP | Non-thermal plasma |
| PEEK | Polyetheretherketone |
| UV | Ultraviolet |
Appendix A


References
- Henningsen, A.; Smeets, R.; Koppen, K.; Sehner, S.; Kornmann, F.; Grobe, A.; Heiland, M.; Gerlach, T. Immediate loading of subcrestally placed dental implants in anterior and premolar sites. J. Craniomaxillofac. Surg. 2017, 45, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, A.; Smeets, R.; Wahidi, A.; Kluwe, L.; Kornmann, F.; Heiland, M.; Gerlach, T. The feasibility of immediately loading dental implants in edentulous jaws. J. Periodontal Implant Sci. 2016, 46, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Madani, E.; Smeets, R.; Freiwald, E.; Sanj, M.S.; Jung, O.; Grubeanu, D.; Hanken, H.; Henningsen, A. Impact of different placement depths on the crestal bone level of immediate versus delayed placed platform-switched implants. J. Craniomaxillofac. Surg. 2018, 46, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Curi, M.M.; Condezo, A.F.B.; Ribeiro, K.; Cardoso, C.L. Long-term success of dental implants in patients with head and neck cancer after radiation therapy. Int. J. Oral Maxillofac. Surg. 2018, 47, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Albrektsson, T. Long-term clinical success of minimally and moderately rough oral implants: A review of 71 studies with 5 years or more of follow-up. Implant Dent. 2015, 24, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Glauser, R.; Schupbach, P.; Gottlow, J.; Hammerle, C.H. Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: A light-microscopic overview and histometric analysis. Clin. Implant Dent. Relat. Res. 2005, 7 (Suppl. 1), S44–S51. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Kim, S.Y.; Rhyu, I.C.; Chung, W.Y.; Leesungbok, R.; Lee, K.W. Influence of microgroove dimension on cell behavior of human gingival fibroblasts cultured on titanium substrata. Clin. Oral. Implants Res. 2009, 20, 56–66. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Pidhatika, B.; Coullerez, G.; Charnley, M.; Kuijer, R.; van der Mei, H.C.; Textor, M.; Busscher, H.J. Bacterial biofilm formation versus mammalian cell growth on titanium-based mono- and bi-functional coating. Eur. Cells Mater. 2010, 19, 205–213. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Sanz-Martin, I.; Sanz-Sanchez, I.; Carrillo de Albornoz, A.; Figuero, E.; Sanz, M. Effects of modified abutment characteristics on peri-implant soft tissue health: A systematic review and meta-analysis. Clin. Oral. Implants Res. 2018, 29, 118–129. [Google Scholar] [CrossRef]
- Xing, R.; Salou, L.; Taxt-Lamolle, S.; Reseland, J.E.; Lyngstadaas, S.P.; Haugen, H.J. Surface hydride on titanium by cathodic polarization promotes human gingival fibroblast growth. J. Biomed. Mater. Res. A 2014, 102, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Shapoff, C.A.; Babushkin, J.A.; Wohl, D.J. Clinical Use of Laser-Microtextured Abutments: A Case Series. Int. J. Periodontics Restor. Dent. 2016, 39, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Al Rezk, F.; Trimpou, G.; Lauer, H.C.; Weigl, P.; Krockow, N. Response of soft tissue to different abutment materials with different surface topographies: A review of the literature. Gen. Dent. 2018, 66, 18–25. [Google Scholar] [PubMed]
- Aita, H.; Att, W.; Ueno, T.; Yamada, M.; Hori, N.; Iwasa, F.; Tsukimura, N.; Ogawa, T. Ultraviolet light-mediated photofunctionalization of titanium to promote human mesenchymal stem cell migration, attachment, proliferation and differentiation. Acta Biomater. 2009, 5, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Hori, N.; Att, W.; Kubo, K.; Iwasa, F.; Ueno, T.; Maeda, H.; Ogawa, T. Ultraviolet treatment overcomes time-related degrading bioactivity of titanium. Tissue Eng. Part A 2009, 15, 3679–3688. [Google Scholar] [CrossRef] [PubMed]
- Henningsen, A.; Smeets, R.; Hartjen, P.; Heinrich, O.; Heuberger, R.; Heiland, M.; Precht, C.; Cacaci, C. Photofunctionalization and non-thermal plasma activation of titanium surfaces. Clin. Oral Investig. 2018, 22, 1045–1054. [Google Scholar] [CrossRef]
- Smeets, R.; Henningsen, A.; Heuberger, R.; Hanisch, O.; Schwarz, F.; Precht, C. Influence of UV Irradiation and Cold Atmospheric Pressure Plasma on Zirconia Surfaces: An In Vitro Study. Int J. Oral Maxillofac. Implants 2019, 34, 329–336. [Google Scholar] [CrossRef]
- Henningsen, A.; Smeets, R.; Heuberger, R.; Jung, O.T.; Hanken, H.; Heiland, M.; Cacaci, C.; Precht, C. Changes in surface characteristics of titanium and zirconia after surface treatment with ultraviolet light or non-thermal plasma. Eur. J. Oral Sci. 2018, 126, 126–134. [Google Scholar] [CrossRef]
- Altmann, B.; Kohal, R.J.; Steinberg, T.; Tomakidi, P.; Bachle-Haas, M.; Wennerberg, A.; Att, W. Distinct cell functions of osteoblasts on UV-functionalized titanium- and zirconia-based implant materials are modulated by surface topography. Tissue Eng. Part C Methods 2013, 19, 850–863. [Google Scholar] [CrossRef]
- Att, W.; Ogawa, T. Biological aging of implant surfaces and their restoration with ultraviolet light treatment: A novel understanding of osseointegration. Int. J. Oral Maxillofac. Implants 2012, 27, 753–761. [Google Scholar]
- Guastaldi, F.P.; Yoo, D.; Marin, C.; Jimbo, R.; Tovar, N.; Zanetta-Barbosa, D.; Coelho, P.G. Plasma treatment maintains surface energy of the implant surface and enhances osseointegration. Int. J. Biomater. 2013, 2013, 354125. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Blanco, C.; Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.A.; Torres-Lagares, D.; Gutierrez-Perez, J.L. Human Osteoblast Cell Behaviour on Titanium Discs Treated with Argon Plasma. Materials 2019, 12, 1735. [Google Scholar] [CrossRef] [PubMed]
- Ozkurt, Z.; Kazazoglu, E. Zirconia dental implants: A literature review. J. Oral Implantol. 2011, 37, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Goutam, M.; Giriyapura, C.; Mishra, S.K.; Gupta, S. Titanium allergy: A literature review. Indian J. Dermatol. 2014, 59, 630. [Google Scholar] [CrossRef]
- Naveau, A.; Rignon-Bret, C.; Wulfman, C. Zirconia abutments in the anterior region: A systematic review of mechanical and esthetic outcomes. J. Prosthet. Dent. 2019, 121, 775–781. [Google Scholar] [CrossRef]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef]
- Georgiev, J.; Vlahova, A.; Kissov, H.; Aleksandrov, S.; Kazakova, R. Possible application of BioHPP in prosthetic dentistry: A literature review. J. IMAB 2018, 24, 1896–1898. [Google Scholar] [CrossRef]
- Atkinson, J.R.; Hay, J.N.; Jenkins, M.J. Enthalpic relaxation in semi-crystalline PEEK. Polymer 2002, 43, 731–735. [Google Scholar] [CrossRef]
- Wiesli, M.G.; Ozcan, M. High-Performance Polymers and Their Potential Application as Medical and Oral Implant Materials: A Review. Implant Dent. 2015, 24, 448–457. [Google Scholar] [CrossRef]
- Bartold, P.M.; Walsh, L.J.; Narayanan, A.S. Molecular and cell biology of the gingiva. Periodontol. 2000 2000, 24, 28–55. [Google Scholar] [CrossRef]
- Moon, I.S.; Berglundh, T.; Abrahamsson, I.; Linder, E.; Lindhe, J. The barrier between the keratinized mucosa and the dental implant. An experimental study in the dog. J. Clin. Periodontol. 1999, 26, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Van den Brand, D.; Massuger, L.F.; Brock, R.; Verdurmen, W.P. Mimicking Tumors: Toward More Predictive In Vitro Models for Peptide- and Protein-Conjugated Drugs. Bioconjug. Chem. 2017, 28, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Schwitalla, A.; Muller, W.D. PEEK dental implants: A review of the literature. J. Oral Implantol. 2013, 39, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Richardson, J.; Lundgren, T. Comparative soft and hard tissue responses to titanium and polymer healing abutments. J. Oral Implantol. 2011, 37, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Giancotti, F.G. Complexity and specificity of integrin signalling. Nat. Cell Biol. 2000, 2, E13–E14. [Google Scholar] [CrossRef]
- Es-Souni, M.; Es-Souni, M.; Fischer-Brandies, H. Assessing the biocompatibility of NiTi shape memory alloys used for medical applications. Anal. Bioanal. Chem. 2005, 381, 557–567. [Google Scholar] [CrossRef]
- Kaivosoja, E.; Barreto, G.; Levon, K.; Virtanen, S.; Ainola, M.; Konttinen, Y.T. Chemical and physical properties of regenerative medicine materials controlling stem cell fate. Ann. Med. 2012, 44, 635–650. [Google Scholar] [CrossRef]
- Aita, H.; Hori, N.; Takeuchi, M.; Suzuki, T.; Yamada, M.; Anpo, M.; Ogawa, T. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials 2009, 30, 1015–1025. [Google Scholar] [CrossRef]
- Lee, J.B.; Jo, Y.H.; Choi, J.Y.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Yeo, I.L. The Effect of Ultraviolet Photofunctionalization on a Titanium Dental Implant with Machined Surface: An In Vitro and In Vivo Study. Materials 2019, 12, 2078. [Google Scholar] [CrossRef]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of osteoblast adhesion to UV-photofunctionalized titanium via an electrostatic mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef] [PubMed]
- Brezavscek, M.; Fawzy, A.; Bachle, M.; Tuna, T.; Fischer, J.; Att, W. The Effect of UV Treatment on the Osteoconductive Capacity of Zirconia-Based Materials. Materials 2016, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhou, J.; Liu, X.; Zheng, M.; Yang, J.; Tan, J. Ultraviolet light-treated zirconia with different roughness affects function of human gingival fibroblasts in vitro: The potential surface modification developed from implant to abutment. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Castellote, M.; Bengtsson, N. Principles of TiO2 photocatalysis. In Application of Titanium Dioxide Photocatalysis to Construction Materials; Ohama, Y., van Gemert, D., Eds.; Springer: Heidelberg, Germany, 2011; Volume 5, pp. 5–10. [Google Scholar]
- Lin, A.; Truong, B.; Patel, S.; Kaushik, N.; Choi, E.H.; Fridman, G.; Fridman, A.; Miller, V. Nanosecond-Pulsed DBD Plasma-Generated Reactive Oxygen Species Trigger Immunogenic Cell Death in A549 Lung Carcinoma Cells through Intracellular Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Wang, X.Z.; Kwon, T.; Huynh, D.L.; Chandimali, N.; Kim, N.; Kang, T.Y.; Ghosh, M.; Gera, M.; Lee, S.B.; et al. Innovative Approach of Non-Thermal Plasma Application for Improving the Growth Rate in Chickens. Int. J. Mol. Sci. 2018, 19, 2301. [Google Scholar] [CrossRef]
- Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef]
- Abu-Sirhan, S.; Hertel, M.; Preissner, S.; Wirtz, H.C.; Herbst, S.R.; Pierdzioch, P.; Raguse, J.D.; Hartwig, S. Bactericidal efficacy of cold plasma in processed bone. A new approach for adjuvant therapy of medication-related osteonecrosis of the jaw? Clin. Plasma Med. 2016, 4, 9–13. [Google Scholar] [CrossRef]
- Daeschlein, G.; Napp, M.; Majumdar, A.; Richter, E.; Rusch-Gerdes, S.; Aly, F.; von Podewils, S.; Sicher, C.; Haase, H.; Niggemeier, M.; et al. In vitro killing of mycobacteria by low temperature atmospheric pressure plasma and dielectric barrier discharge plasma for treatment of tuberculosis. Clin. Plasma Med. 2017, 5–6, 1–7. [Google Scholar] [CrossRef]
- Kang, S.U.; Kim, Y.S.; Kim, Y.E.; Park, J.K.; Lee, Y.S.; Kang, H.Y.; Jang, J.W.; Ryeo, J.B.; Lee, Y.; Shin, Y.S.; et al. Opposite effects of non-thermal plasma on cell migration and collagen production in keloid and normal fibroblasts. PLoS ONE 2017, 12, e0187978. [Google Scholar] [CrossRef]
- Zheng, M.; Zhan, L.L.; Liu, Z.Q.; Li, H.P.; Tan, J.G. Effect of different plasma treated zirconia on the adhensive behaviour of human gingival fibroblasts. Beijing Da Xue Xue Bao Yi Xue Ban 2019, 51, 315–320. [Google Scholar]
- Lee, J.H.; Kim, Y.H.; Choi, E.H.; Kim, K.M.; Kim, K.N. Air atmospheric-pressure plasma-jet treatment enhances the attachment of human gingival fibroblasts for early peri-implant soft tissue seals on titanium dental implant abutments. Acta Odontol. Scand. 2015, 73, 67–75. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Smeets, R.; Kluwe, L.; Hartjen, P.; Barbeck, M.; Cacaci, C.; Gosau, M.; Henningsen, A. Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma. Int. J. Mol. Sci. 2019, 20, 5596. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225596
Guo L, Smeets R, Kluwe L, Hartjen P, Barbeck M, Cacaci C, Gosau M, Henningsen A. Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma. International Journal of Molecular Sciences. 2019; 20(22):5596. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225596
Chicago/Turabian StyleGuo, Linna, Ralf Smeets, Lan Kluwe, Philip Hartjen, Mike Barbeck, Claudio Cacaci, Martin Gosau, and Anders Henningsen. 2019. "Cytocompatibility of Titanium, Zirconia and Modified PEEK after Surface Treatment Using UV Light or Non-Thermal Plasma" International Journal of Molecular Sciences 20, no. 22: 5596. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225596