DEK Is a Potential Biomarker Associated with Malignant Phenotype in Gastric Cancer Tissues and Plasma
Abstract
:1. Introduction
2. Results
2.1. Identification and Validation Studies for DEK, a Potential Biomarker for GC
2.2. Clinicopathologic Correlations of DEK in Gastric Tissues by IHC Study
2.3. Correlation of Plasma DEK with Clinicopathological Characteristic and Survival Outcome in GC Patients
2.4. Plasma DEK as a Potential Diagnostic Biomarker in GC Patients
2.5. DEK Is Involved in the Invasive Ability of GC Cell Lines
2.6. DEK Promotes GC Cell Metastasis Mediated by MMP-2 and MMP-9
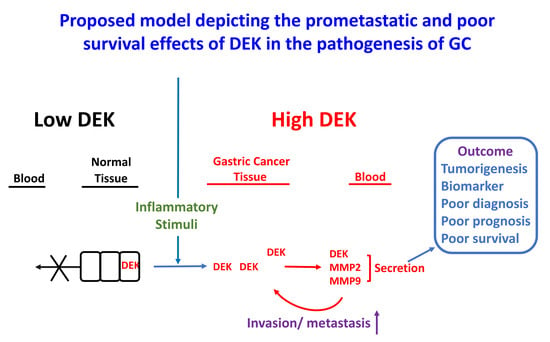
3. Discussion
4. Materials and Methods
4.1. Clinical Specimens
4.2. Statistical Analysis
4.3. Cell Culture
4.4. Measurement of CEA, CA19.9, and CRP Levels
4.5. qRT-PCR
4.6. Western Blot
4.7. ELISA
4.8. Overexpression/Depletion of DEK in GC Cell Lines
4.9. In Vitro Invasion Assay
4.10. Immunohistochemistry (IHC) and Scoring
4.11. Gelatin Zymography
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CA 19.9 | Carbohydrate antigen 19.9 |
| CEA | Carcinoembryonic antigen |
| CGMH | Chang Gung Memorial Hospital |
| CI | Confidence interval |
| CRP | C-reactive protein |
| ELISA | Enzyme-linked immunosorbent assay |
| GC | Gastric cancer |
| Hp | Helicobacter pylori |
| HR | Hazard ratio |
| IHC | Immunohistochemistry |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| MMP | Matrix metalloproteinase |
| ROC | Receiver operating characteristic |
| TNM stage | Tumor–node–metastasis stage |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA A Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare, Taiwan. The Ten Leading Cause of Death 2018. Available online: https://www.mohw.gov.tw/cp-16-48057-1.html (accessed on 10 September 2019).
- Chen, X.Z.; Zhang, W.H.; Yang, K.; Zhang, B.; Chen, Z.X.; Chen, J.P.; Zhou, Z.G.; Hu, J.K. Quantitative comparisons of summary receiver operating characteristics (sROC) curves among conventional serological tumor biomarkers for predicting gastric cancer in Chinese population. Tumour Biol. 2014, 35, 9015–9022. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, D.; Roviello, F.; De Stefano, A.; Farnetani, M.; Garosi, L.; Messano, A.; Pinto, E. Prognostic significance of CEA, CA 19-9 and CA 72-4 preoperative serum levels in gastric carcinoma. Oncology 1999, 57, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Sun, C.F.; Pai, H.J.; Wang, W.K.; Hsieh, C.C.; Kuo, L.M.; Wang, C.S. Preoperative serum C-reactive protein and gastric cancer; clinical-pathological correlation and prognostic significance. Chang. Gung Med. J. 2010, 33, 301–312. [Google Scholar] [PubMed]
- Wang, J.; Yu, J.C.; Kang, W.M.; Ma, Z.Q. Treatment strategy for early gastric cancer. Surg. Oncol. 2012, 21, 119–123. [Google Scholar] [CrossRef]
- Nanjappa, V.; Thomas, J.K.; Marimuthu, A.; Muthusamy, B.; Radhakrishnan, A.; Sharma, R.; Ahmad Khan, A.; Balakrishnan, L.; Sahasrabuddhe, N.A.; Kumar, S.; et al. Plasma Proteome Database as a resource for proteomics research: 2014 update. Nucleic Acids Res. 2014, 42, D959–D965. [Google Scholar] [CrossRef]
- Broxmeyer, H.E.; Mor-Vaknin, N.; Kappes, F.; Legendre, M.; Saha, A.K.; Ou, X.; O’Leary, H.; Capitano, M.; Cooper, S.; Markovitz, D.M. Concise review: Role of DEK in stem/progenitor cell biology. Stem Cells 2013, 31, 1447–1453. [Google Scholar] [CrossRef]
- Sun, J.; Bi, F.; Yang, Y.; Zhang, Y.; Jin, A.; Li, J.; Lin, Z. DEK protein overexpression predicts poor prognosis in pancreatic ductal adenocarcinoma. Oncol. Rep. 2017, 37, 857–864. [Google Scholar] [CrossRef]
- Chen, X.; Wu, X.; Ouyang, W.; Gu, M.; Gao, Z.; Song, M.; Chen, Y.; Lin, Y.; Cao, Y.; Xiao, H. Novel ent-Kaurane Diterpenoid from Rubus corchorifolius L. f. Inhibits Human Colon Cancer Cell Growth via Inducing Cell Cycle Arrest and Apoptosis. J. Agric. Food Chem. 2017, 65, 1566–1573. [Google Scholar] [CrossRef]
- Datta, A.; Adelson, M.E.; Mogilevkin, Y.; Mordechai, E.; Sidi, A.A.; Trama, J.P. Oncoprotein DEK as a tissue and urinary biomarker for bladder cancer. BMC Cancer 2011, 11, 234. [Google Scholar] [CrossRef]
- Adams, A.K.; Hallenbeck, G.E.; Casper, K.A.; Patil, Y.J.; Wilson, K.M.; Kimple, R.J.; Lambert, P.F.; Witte, D.P.; Xiao, W.; Gillison, M.L.; et al. DEK promotes HPV-positive and -negative head and neck cancer cell proliferation. Oncogene 2015, 34, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zou, L.; Yao, Q.; Zhang, Y.; Gan, L.; Tang, L. Silencing DEK downregulates cervical cancer tumorigenesis and metastasis via the DEK/p-Ser9-GSK-3beta/p-Tyr216-GSK-3beta/beta-catenin axis. Oncol. Rep. 2017, 38, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liang, Z.; Li, C.; Yang, Z.; Chen, L. LCMR1 interacts with DEK to suppress apoptosis in lung cancer cells. Mol. Med. Rep. 2017, 16, 4159–4164. [Google Scholar] [CrossRef] [PubMed]
- Secchiero, P.; Voltan, R.; di Iasio, M.G.; Melloni, E.; Tiribelli, M.; Zauli, G. The oncogene DEK promotes leukemic cell survival and is downregulated by both Nutlin-3 and chlorambucil in B-chronic lymphocytic leukemic cells. Clin. Cancer Res. 2010, 16, 1824–1833. [Google Scholar] [CrossRef]
- Piao, J.; Shang, Y.; Liu, S.; Piao, Y.; Cui, X.; Li, Y.; Lin, Z. High expression of DEK predicts poor prognosis of gastric adenocarcinoma. Diagn. Pathol. 2014, 9, 67. [Google Scholar] [CrossRef]
- Yu, L.; Huang, X.; Zhang, W.; Zhao, H.; Wu, G.; Lv, F.; Shi, L.; Teng, Y. Critical role of DEK and its regulation in tumorigenesis and metastasis of hepatocellular carcinoma. Oncotarget 2016, 7, 26844–26855. [Google Scholar] [CrossRef]
- Caliskaner, Z.O.; Cakar, T.; Ozcelik, E.; Ozdilek, A.; Kim, A.S.; Dogan, O.; Bosompem, A.; Grosveld, G.; Saka, B.; Kandilci, A. DEK protein level is a biomarker of CD138positive normal and malignant plasma cells. PLoS ONE 2017, 12, e0178025. [Google Scholar] [CrossRef]
- Bub, C.B.; Torres, M.A.; Velloso, E.D.; Silveira, P.A.; Correia, R.P.; Bacal, N.S.; Mangueira, C.L.; Fernandes, J.F.; Odone Filho, V.; Hamerschlak, N.; et al. Eosinophil chimerism in the differential diagnosis between DEK-NUP214-positive acute myeloid leukaemia relapse and chronic graft-versus-host disease. J. Clin. Pathol. 2015, 68, 950–952. [Google Scholar]
- Riveiro-Falkenbach, E.; Ruano, Y.; Garcia-Martin, R.M.; Lora, D.; Cifdaloz, M.; Acquadro, F.; Ballestin, C.; Ortiz-Romero, P.L.; Soengas, M.S.; Rodriguez-Peralto, J.L. DEK oncogene is overexpressed during melanoma progression. Pigment Cell Melanoma Res. 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Khodadoust, M.S.; Verhaegen, M.; Kappes, F.; Riveiro-Falkenbach, E.; Cigudosa, J.C.; Kim, D.S.; Chinnaiyan, A.M.; Markovitz, D.M.; Soengas, M.S. Melanoma proliferation and chemoresistance controlled by the DEK oncogene. Cancer Res. 2009, 69, 6405–6413. [Google Scholar] [CrossRef]
- Choi, S.; Park, S.Y.; Jeong, J.; Cho, E.; Phark, S.; Lee, M.; Kwak, D.; Lim, J.Y.; Jung, W.W.; Sul, D. Identification of toxicological biomarkers of di(2-ethylhexyl) phthalate in proteins secreted by HepG2 cells using proteomic analysis. Proteomics 2010, 10, 1831–1846. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Park, S.Y.; Kwak, D.; Phark, S.; Lee, M.; Lim, J.Y.; Jung, W.W.; Sul, D. Proteomic analysis of proteins secreted by HepG2 cells treated with butyl benzyl phthalate. J. Toxicol. Environ. Health Part A 2010, 73, 1570–1585. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Michelis, M.A.; Wang, J.; Bose, R.; DeLange, T.; Reeves, W.H. Autoantibodies to DEK oncoprotein in a patient with systemic lupus erythematosus and sarcoidosis. Arthritis Rheum. 1998, 41, 1505–1510. [Google Scholar] [CrossRef]
- Murray, K.J.; Szer, W.; Grom, A.A.; Donnelly, P.; Levinson, J.E.; Giannini, E.H.; Glass, D.N.; Szer, I.S. Antibodies to the 45 kDa DEK nuclear antigen in pauciarticular onset juvenile rheumatoid arthritis and iridocyclitis: Selective association with MHC gene. J. Rheumatol. 1997, 24, 560–567. [Google Scholar] [PubMed]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Faulkner, N.; Legendre, M.; Khodadoust, M.S.; Kappes, F.; Ruth, J.H.; Koch, A.; Glass, D.; et al. The DEK nuclear autoantigen is a secreted chemotactic factor. Mol. Cell. Biol. 2006, 26, 9484–9496. [Google Scholar] [CrossRef]
- Hoeboer, S.H.; van der Geest, P.J.; Nieboer, D.; Groeneveld, A.B. The diagnostic accuracy of procalcitonin for bacteraemia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2015, 21, 474–481. [Google Scholar] [CrossRef]
- Pease, N.A.; Wise-Draper, T.; Privette Vinnedge, L. Dissecting the Potential Interplay of DEK Functions in Inflammation and Cancer. J. Oncol. 2015, 2015, 106517. [Google Scholar] [CrossRef]
- Zheng, T.H.; Zhao, J.L.; Guleng, B. Advances in Molecular Biomarkers for Gastric Cancer. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 299–305. [Google Scholar] [CrossRef]
- Huang, Z.; Ma, L.; Huang, C.; Li, Q.; Nice, E.C. Proteomic profiling of human plasma for cancer biomarker discovery. Proteomics 2017, 17, 1600240. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, W.; Fang, C.; Raj, S.S.; Hu, W.M.; Li, Q.W.; Zhou, Z.W. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget 2016, 7, 49565–49573. [Google Scholar] [CrossRef]
- Liu, K.; Feng, T.; Liu, J.; Zhong, M.; Zhang, S. Silencing of the DEK gene induces apoptosis and senescence in CaSki cervical carcinoma cells via the up-regulation of NF-kappaB p65. Biosci. Rep. 2012, 32, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Sanden, C.; Gullberg, U. The DEK oncoprotein and its emerging roles in gene regulation. Leukemia 2015, 29, 1632–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannetti, A.; Ledoux, A.C.; Tudhope, S.J.; Sellier, H.; Zhao, B.; Mowla, S.; Moore, A.; Hummerich, H.; Gewurz, B.E.; Cockell, S.J.; et al. Regulation of p53 and Rb links the alternative NF-kappaB pathway to EZH2 expression and cell senescence. PLoS Genet. 2014, 10, e1004642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sun, L.; Yang, M.; Luo, W.; Gao, Y.; Liu, Z.; Qiu, X.; Wang, E. DEK depletion negatively regulates Rho/ROCK/MLC pathway in non-small cell lung cancer. J. Histochem. Cytochem. 2013, 61, 510–521. [Google Scholar] [CrossRef] [Green Version]
- Wise-Draper, T.; Sendilnathan, A.; Palackdharry, S.; Pease, N.; Qualtieri, J.; Butler, R.; Sadraei, N.H.; Morris, J.C.; Patil, Y.; Wilson, K.; et al. Decreased plasma DEK Oncogene Levels Correlate with p16-Negative Disease and Advanced Tumor Stage in a Case-Control Study of Patients with Head and Neck Squamous Cell Carcinoma. Transl. Oncol. 2018, 11, 168–174. [Google Scholar] [CrossRef]
- Mor-Vaknin, N.; Rivas, M.; Legendre, M.; Mohan, S.; Yuanfan, Y.; Mau, T.; Johnson, A.; Huang, B.; Zhao, L.; Kimura, Y.; et al. High levels of DEK autoantibodies in sera of polyarticular JIA patients and in early flare following cessation of anti-TNF therapy. Arthritis Rheumatol. 2017, 70, 594. [Google Scholar] [CrossRef] [Green Version]
- Kappes, F.; Fahrer, J.; Khodadoust, M.S.; Tabbert, A.; Strasser, C.; Mor-Vaknin, N.; Moreno-Villanueva, M.; Burkle, A.; Markovitz, D.M.; Ferrando-May, E. DEK is a poly(ADP-ribose) acceptor in apoptosis and mediates resistance to genotoxic stress. Mol. Cell. Biol. 2008, 28, 3245–3257. [Google Scholar] [CrossRef] [Green Version]
- You, S.; Guan, Y.; Li, W. Epithelialmesenchymal transition in colorectal carcinoma cells is mediated by DEK/IMP3. Mol. Med. Rep. 2018, 17, 1065–1070. [Google Scholar]
- Sadeeshkumar, V.; Duraikannu, A.; Ravichandran, S.; Kodisundaram, P.; Fredrick, W.S.; Gobalakrishnan, R. Modulatory efficacy of dieckol on xenobiotic-metabolizing enzymes, cell proliferation, apoptosis, invasion and angiogenesis during NDEA-induced rat hepatocarcinogenesis. Mol. Cell. Biochem. 2017, 433, 195–204. [Google Scholar] [CrossRef]
- Hui, W.; Ma, X.; Zan, Y.; Song, L.; Zhang, S.; Dong, L. MicroRNA-1292-5p inhibits cell growth, migration and invasion of gastric carcinoma by targeting DEK. Am. J. Cancer Res. 2018, 8, 1228–1238. [Google Scholar]
- McIntosh, M.W.; Drescher, C.; Karlan, B.; Scholler, N.; Urban, N.; Hellstrom, K.E.; Hellstrom, I. Combining CA 125 and SMR serum markers for diagnosis and early detection of ovarian carcinoma. Gynecol. Oncol. 2004, 95, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.M.; Wang, C.S.; Tsai, C.Y.; Chen, C.Y.; Chi, H.C.; Tseng, Y.H.; Chung, P.J.; Lin, Y.H.; Chung, I.H.; Lin, K.H. MicroRNA-196a/-196b promote cell metastasis via negative regulation of radixin in human gastric cancer. Cancer Lett. 2014, 351, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.L.; Tsai, M.M.; Tsai, C.Y.; Huang, Y.H.; Chen, C.Y.; Chi, H.C.; Tseng, Y.H.; Chao, I.W.; Lin, W.C.; Wu, S.M.; et al. Glyoxalase-I is a novel prognosis factor associated with gastric cancer progression. PLoS ONE 2012, 7, e34352. [Google Scholar] [CrossRef]
- Tsai, M.M.; Lin, P.Y.; Cheng, W.L.; Tsai, C.Y.; Chi, H.C.; Chen, C.Y.; Tseng, Y.H.; Cheng, Y.F.; Chen, C.D.; Liang, Y.; et al. Overexpression of ADP-ribosylation factor 1 in human gastric carcinoma and its clinicopathological significance. Cancer Sci. 2012, 103, 1136–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.M.; Huang, H.W.; Wang, C.S.; Lee, K.F.; Tsai, C.Y.; Lu, P.H.; Chi, H.C.; Lin, Y.H.; Kuo, L.M.; Lin, K.H. MicroRNA-26b inhibits tumor metastasis by targeting the KPNA2/c-jun pathway in human gastric cancer. Oncotarget 2016, 7, 39511–39526. [Google Scholar] [CrossRef]
- Chi, H.C.; Chen, S.L.; Liao, C.J.; Liao, C.H.; Tsai, M.M.; Lin, Y.H.; Huang, Y.H.; Yeh, C.T.; Wu, S.M.; Tseng, Y.H.; et al. Thyroid hormone receptors promote metastasis of human hepatoma cells via regulation of TRAIL. Cell Death Differ. 2012, 19, 1802–1814. [Google Scholar] [CrossRef] [Green Version]





| Characteristics | No. | High Expression a No. (%) | p-Value b | 5-yr S.R. c | Log-Rank p d |
|---|---|---|---|---|---|
| Age (yrs) | |||||
| <65 | 40 | 27 (67.5%) | 1.000 | 51.6 | 0.5487 |
| ≥65 | 52 | 36 (69.2%) | 55.4 | ||
| Gender | |||||
| Male | 51 | 36 (70.6%) | 0.6574 | 54.0 | 0.9059 |
| Female | 41 | 27 (65.9%) | 53.4 | ||
| Location | |||||
| Upper third | 21 | 14 (66.7%) | 0.5558 | 57.4 | 0.9837 f |
| Middle third | 21 | 12 (57.1%) | 58.7 | ||
| Lower third | 45 | 33 (73.3%) | 56.1 | ||
| Whole | 5 | 4 (80.0%) | 0.0 | ||
| Gross type | |||||
| Localized | 36 | 15 (41.7%) | <0.0001 | 82.1 | <0.0001 |
| Infiltrative | 56 | 48 (85.7%) | 34.5 | ||
| Size (maximal diameter) | |||||
| <5 cm | 51 | 26 (51.0%) | <0.0001 | 77.2 | <0.0001 |
| ≥5 cm | 41 | 37 (90.2%) | 20.3 | ||
| Histological type | |||||
| Intestinal | 26 | 14 (53.8%) | 0.0809 | 82.9 | 0.0011 |
| Diffuse | 66 | 49 (72.4%) | 40.2 | ||
| Depth of invasion (pT) | |||||
| T1 | 19 | 2 (10.5%) | <0.0001 | 94.1 | <0.0001 |
| T2 | 13 | 9 (69.2%) | 72.5 | ||
| T3 | 41 | 35 (85.4%) | 42.7 | ||
| T4 | 19 | 17 (89.5%) | 7.9 | ||
| Serosal invasion | |||||
| No (T1, T2) | 32 | 11 (34.4%) | <0.0001 | 85.9 | <0.0001 |
| Yes (T3, T4) | 60 | 52 (86.7%) | 31.9 | ||
| Lymph node status (pN) | |||||
| N0 | 30 | 9 (30.0%) | <0.0001 | 93.1 | <0.0001 |
| N1 | 34 | 28 (82.4%) | 48.0 | ||
| N2 | 18 | 16 (88.9%) | 17.1 | ||
| N3 | 10 | 10 (100.0%) | 0.0 | ||
| Lymph node metastasis | |||||
| No | 30 | 9 (30.0%) | <0.0001 | 93.1 | <0.0001 |
| Yes | 62 | 54 (87.1%) | 32.1 | ||
| Distant metastasis (pM) | |||||
| No | 75 | 46 (61.3%) | 0.0010 | 67.2 | <0.0001 |
| Yes | 17 | 17 (100.0%) | 0.0 | ||
| Pathological stage (pStage) | |||||
| Stage I | 23 | 4 (17.4%) | <0.0001 | 100.0 | <0.0001 |
| Stage II | 12 | 7 (58.3%) | 68.8 | ||
| Stage III | 37 | 33 (89.2%) | 42.3 | ||
| Stage IV | 20 | 9 (95.0%) | 0.0 | ||
| Pathological stage | |||||
| Stage I, II | 35 | 11 (31.4%) | <0.0001 | 90.1 | <0.0001 |
| Stage III, IV | 57 | 52 (91.2%) | 25.2 | ||
| Liver metastasis | |||||
| No | 89 | 60 (67.4%) | 0.5490 | 55.3 | 0.0030 |
| Yes | 3 | 3 (100.0%) | 0.0 | ||
| Peritoneal seeding | |||||
| No | 77 | 49 (63.6%) | 0.0312 | 65.9 | <0.0001 |
| Yes | 15 | 14 (93.3%) | 0.0 | ||
| Vascular invasion | |||||
| No | 68 | 45 (66.2%) | 0.6099 | 64.7 | 0.0001 |
| Yes | 24 | 18 (75.0%) | 19.9 | ||
| Lymphatic invasion | |||||
| No | 38 | 17 (44.7%) | <0.0001 | 79.6 | <0.0001 |
| Yes | 54 | 46 (85.2%) | 35.7 | ||
| Perineural invasion | |||||
| No | 52 | 30 (57.7%) | 0.0133 | 68.8 | 0.0008 |
| Yes | 40 | 33 (82.5%) | 32.4 | ||
| DEK (e) (IHC) expression | |||||
| Low | 29 | 81.7 | 0.0004 | ||
| High | 63 | 40.0 | |||
| Tissues | No. Patients | DEK Staining Score a/No. Patients (%) | |||
|---|---|---|---|---|---|
| - | + | ++ | +++ | ||
| Tumor | 92 | 0 | 0 | 29 (31.5%) | 63 (68.5%) |
| Adjacent mucosa | 90 | 0 | 2 (2.2%) | 88 (97.8%) | 0 |
| Clinicopathological Correlations | Univariate Analysis | ||||
|---|---|---|---|---|---|
| Characteristics | No. | Mean ± SE Plasma DEK (pg/mL) a | pb | 5-yr S.R. c | Log-Rank p d |
| Age (yrs) | |||||
| <65 | 45 | 762.4 ± 79.1 | 0.4205 | 60.1 | 0.2335 |
| ≥65 | 53 | 820.7 ± 69.3 | 67.2 | ||
| Gender | |||||
| Male | 56 | 751.3 ± 61.5 | 0.5346 | 69.8 | 0.1061 |
| Female | 42 | 850.8 ± 88.6 | 56.4 | ||
| Location | |||||
| Upper third | 21 | 816.2 ± 105.2 | 0.7472 | 54.6 | 0.3590 (f) |
| Middle third | 23 | 795.1 ± 120.5 | 75.6 | ||
| Lower third | 50 | 770.0 ± 71.6 | 68.7 | ||
| Whole | 4 | 987.5 ± 283.4 | 0.0 | ||
| Gross type | |||||
| Localized | 47 | 584.3 ± 78.1 | <0.0001 | 86.4 | <0.0001 |
| Infiltrative | 51 | 987.1 ± 58.0 | 42.7 | ||
| Size (maximal diameter) | |||||
| <5 cm | 55 | 613.6 ± 57.3 | <0.0001 | 84.8 | <0.0001 |
| ≥5 cm | 43 | 1024.5 ± 81.2 | 34.3 | ||
| Histological type | |||||
| Intestinal | 28 | 554.9 ± 72.4 | 0.0058 | 92.6 | 0.0016 |
| Diffuse | 70 | 889.5 ± 63.5 | 53.2 | ||
| Depth of invasion (pT) | |||||
| T1 | 24 | 311.4 ± 58.6 | <0.0001 | 93.3 | <0.0001 |
| T2 | 17 | 774.2 ± 151.8 | 86.3 | ||
| T3 | 42 | 909.8 ± 61.1 | 58.0 | ||
| T4 | 15 | 1263.6 ± 136.6 | 0.0 | ||
| Serosal invasion | |||||
| No (T1, T2) | 41 | 503.3 ± 76.9 | <0.0001 | 90.5 | <0.0001 |
| Yes (T3, T4) | 57 | 102.9 ± 56.2 | 42.1 | ||
| Lymph node status (pN) | |||||
| N0 | 37 | 505.8 ± 80.2 | <0.0001 | 91.9 | <0.0001 |
| N1 | 36 | 893.9 ± 70.0 | 65.9 | ||
| N2 | 17 | 1065.1 ± 126.0 | 21.6 | ||
| N3 | 8 | 1100.1 ± 148.8 | 14.3 | ||
| Lymph node metastasis | |||||
| No | 37 | 505.8 ± 80.2 | <0.0001 | 91.9 | 0.0001 |
| Yes | 61 | 868.7 ± 57.7 | 46.3 | ||
| Distant metastasis (pM) | |||||
| No | 81 | 674.7 ± 49.8 | <0.0001 | 77.0 | <0.0001 |
| Yes | 17 | 1362.1 ± 105.7 | 0.0 | ||
| Pathological stage (pStage) | |||||
| Stage I | 30 | 375.2 ± 79.3 | <0.0001 | 100.0 | <0.0001 |
| Stage II | 12 | 752.4 ± 150.4 | 75.8 | ||
| Stage III | 37 | 872.7 ± 44.5 | 54.0 | ||
| Stage IV | 19 | 1327.8 ± 104.1 | 0.0 | ||
| Pathological stage | |||||
| Stage I, II | 42 | 483.0 ± 75.0 | <0.0001 | 92.6 | <0.0001 |
| Stage III, IV | 56 | 1027.1 ± 53.9 | 35.6 | ||
| Liver metastasis | |||||
| No | 97 | 968.1 ± 52.2 | 0.2095 | 64.9 | 0.0022 |
| Yes | 1 | 1354.1 | 0.0 | ||
| Peritoneal seeding | |||||
| No | 83 | 717.4 ± 53.3 | 0.0006 | 74.0 | <0.0001 |
| Yes | 15 | 1217.3 ± 123.9 | 14.3 | ||
| Vascular invasion | |||||
| No | 71 | 678.8 ± 57.3 | 0.0003 | 75.0 | 0.0001 |
| Yes | 27 | 1096.5 ± 92.5 | 34.4 | ||
| Lymphatic invasion | |||||
| No | 43 | 576.0 ± 71.3 | 0.0001 | 88.1 | 0.0001 |
| Yes | 55 | 964.2 ± 65.9 | 44.7 | ||
| Perineural invasion | |||||
| No | 54 | 600.3 ± 64.4 | <0.0001 | 80.7 | 0.0001 |
| Yes | 44 | 1031.5 ± 72.3 | 42.1 | ||
| DEK (Plasma) | |||||
| <median (e) (=734.0 pg/mL) | 49 | 88.7 | <0.0001 | ||
| ≥median | 49 | 34.2 | |||
| CEA (Plasma) | |||||
| <median (=2.11 ng/mL) | 49 | 57.1 | 0.1442 | ||
| ≥median | 49 | 67.6 | |||
| CA19.9 (Plasma) | |||||
| <median (=9.91 U/mL) | 49 | 70.2 | 0.3420 | ||
| ≥median | 49 | 57.9 | |||
| CRP (Plasma) | |||||
| <median (=1.79 mg/L) | 49 | 83.9 | <0.0001 | ||
| ≥median | 49 | 41.7 | |||
| Characteristics | B a | SE b | Wald | HR c | 95% CI d | P e |
|---|---|---|---|---|---|---|
| Histological type (intestinal/diffuse) | 1.341 | 1.113 | 1.451 | 3.824 | 0.431–33.902 | 0.228 |
| Gross type (localized/infiltrative) | 0.442 | 0.661 | 0.446 | 1.555 | 0.425–5.687 | 0.504 |
| Tumor size (<5 cm/≥5 cm) | 0.644 | 0.653 | 0.974 | 1.905 | 0.530–6.851 | 0.324 |
| Serosal invasion (no/yes) | −0.085 | 0.820 | 0.011 | 0.918 | 0.184–4.581 | 0.917 |
| Lymph node metastasis (no/yes) | 0.176 | 1.072 | 0.027 | 1.192 | 0.146–9.742 | 0.870 |
| Distant metastasis (no/yes) | 0.102 | 0.623 | 0.027 | 1.107 | 0.326–3.754 | 0.871 |
| Liver metastasis (no/yes) | 3.363 | 1.848 | 3.312 | 28.887 | 0.772–1081.15 | 0.069 |
| Pathological stage (I, II/III, IV) | 1.375 | 1.096 | 1.573 | 3.955 | 0.461–33.926 | 0.210 |
| Peritoneal invasion (no/yes) | 0.301 | 0.668 | 0.203 | 1.351 | 0.365–5.007 | 0.652 |
| Vascular invasion (no/yes) | 0.268 | 0.464 | 0.333 | 1.307 | 0.526–3.247 | 0.564 |
| Lymphatic invasion (no/yes) | −0.040 | 0.717 | 0.003 | 0.961 | 0.236–3.920 | 0.956 |
| Perineural invasion (no/yes) | 0.265 | 0.506 | 0.274 | 1.303 | 0.484–3.511 | 0.601 |
| CRP (<median/≥median) | 0.311 | 0.500 | 0.387 | 1.365 | 0.512–3.639 | 0.534 |
| Plasma DEK level (<median/≥median) | 1.119 | 0.532 | 4.421 | 3.061 | 1.079–8.682 | 0.035 |
| Biomarkers | AUC a | SE b | p-Value c | Cut-Off Value | Sensitivity d | Specificity e |
|---|---|---|---|---|---|---|
| DEK | 0.797 | 0.031 | <0.001 | 484.22 pg/mL | 70.4% | 79.0% |
| CEA | 0.770 | 0.033 | <0.001 | 1.90 ng/mL | 66.3% | 80.0% |
| CA19.9 | 0.555 | 0.042 | 0.1726 | 23.57 U/mL | 27.6% | 95.8% |
| CRP | 0.593 | 0.041 | 0.0228 | 1.81 mg/L | 50.0% | 74.2% |
| Biomarkers | AUC a | Sensitivity b | Specificity b |
|---|---|---|---|
| DEK vs. CEA | 0.5493 | 0.5388 | 0.8573 |
| DEK vs. CA19.9 | <0.0001 | <0.0001 | 0.0003 |
| DEK vs. CRP | <0.0001 | 0.0035 | 0.4819 |
| CEA vs. CA19.9 | <0.0001 | <0.0001 | 0.0004 |
| CEA vs. CRP | 0.0005 | 0.0205 | 0.3568 |
| CA19.9 vs. CRP | 0.4911 | 0.0013 | <0.0001 |
| Plasma Biomarkers | Upper Limit of Reference Range | Sensitivity | Specificity |
|---|---|---|---|
| DEK | 846.63 pg/μL | 42.9% | 64.1% |
| CEA | 3.65 ng/mL | 23.5% | 64.3% |
| CA19.9 | 23.42 U/mL | 27.6% | 61.6% |
| CRP | 3.62 mg/L | 18.4% | 59.0% |
| Biomarkers | Sensitivity |
|---|---|
| DEK vs. CEA | 0.0063 |
| DEK vs. CA19.9 | 0.0363 |
| DEK vs. CRP | 0.0004 |
| CEA vs. CRP | 0.6250 |
| CEA vs. CA19.9 | 0.4824 |
| CRP vs. CA19.9 | 0.1742 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-F.; Tsai, M.-M.; Tsai, C.-Y.; Huang, C.-G.; Ou, Y.-H.; Hsieh, C.-C.; Hsieh, H.-L.; Wang, C.-S.; Lin, K.-H. DEK Is a Potential Biomarker Associated with Malignant Phenotype in Gastric Cancer Tissues and Plasma. Int. J. Mol. Sci. 2019, 20, 5689. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225689
Lee K-F, Tsai M-M, Tsai C-Y, Huang C-G, Ou Y-H, Hsieh C-C, Hsieh H-L, Wang C-S, Lin K-H. DEK Is a Potential Biomarker Associated with Malignant Phenotype in Gastric Cancer Tissues and Plasma. International Journal of Molecular Sciences. 2019; 20(22):5689. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225689
Chicago/Turabian StyleLee, Kam-Fai, Ming-Ming Tsai, Chung-Ying Tsai, Chung-Guei Huang, Yu-Hsiang Ou, Ching-Chuan Hsieh, Hsi-Lung Hsieh, Chia-Siu Wang, and Kwang-Huei Lin. 2019. "DEK Is a Potential Biomarker Associated with Malignant Phenotype in Gastric Cancer Tissues and Plasma" International Journal of Molecular Sciences 20, no. 22: 5689. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225689