Tellimagrandin II, A Type of Plant Polyphenol Extracted from Trapa bispinosa Inhibits Antibiotic Resistance of Drug-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. TGII Exhibits Potent Antimicrobial Effects Against S. Aureus Strains
2.2. TGII Possesses Potent Bactericidal Activity Against Drug-Resistant S. Aureus Strains
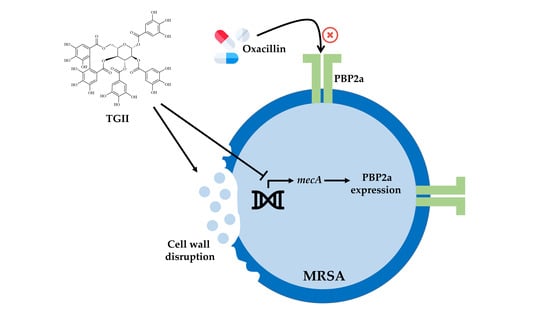
2.3. TGII Reduces the Resistance of MRSA by Regulating PBP2a Expression
2.4. TGII Disrupts the Cell Wall Integrity of MRSA
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Bacterial Strains and PBMCs
4.3. Cell Viability Determination
4.4. MIC Assay
4.5. FIC Determination
4.6. Time-Kill Curve Determination
4.7. Quantitative Real-Time PCR
4.8. Western Blot Analysis
4.9. PBP2a Latex Agglutination Assay
4.10. BOCILLIN FL Assay
4.11. Transmission Electron Microscopy (TEM)
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| TGII | Tellimagrandin II |
| TEM | transmission electron microscopy |
| MRSA | methicillin-resistant Staphylococcus aureus |
| MSSA | methicillin-sensitive Staphylococcus aureus |
| PBP2a | penicillin-binding protein 2a |
| PBMCs | peripheral blood mononuclear cells |
| MH | Mueller-Hinton |
| MIC | minimal inhibitory concentration |
| FIC | fractional inhibitory concentration |
References
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Schleimer, N.; Kaspar, U.; Knaack, D.; von Eiff, C.; Molinaro, S.; Grallert, H.; Idelevich, E.A.; Becker, K. In Vitro Activity of the Bacteriophage Endolysin HY-133 against Staphylococcus aureus Small-Colony Variants and Their Corresponding Wild Types. Int. J. Mol. Sci. 2019, 20, 716. [Google Scholar] [CrossRef]
- Wu, T.H.; Lee, C.Y.; Yang, H.J.; Fang, Y.P.; Chang, Y.F.; Tzeng, S.L.; Lu, M.C. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus among nasal carriage strains isolated from emergency department patients and healthcare workers in central Taiwan. J. Microbiol. Immunol. Infect. 2019, 52, 248–254. [Google Scholar] [CrossRef]
- Tang, K.W.; Yang, S.C.; Tseng, C.H. Design, Synthesis, and Anti-Bacterial Evaluation of Triazolyl-Pterostilbene Derivatives. Int. J. Mol. Sci. 2019, 20, 4564. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Yaw, L.K.; Robinson, J.O.; Ho, K.M. A comparison of long-term outcomes after meticillin-resistant and meticillin-sensitive Staphylococcus aureus bacteraemia: An observational cohort study. Lancet. Infect. Dis. 2014, 14, 967–975. [Google Scholar] [CrossRef]
- Adkar, P.; Dongare, A.; Ambavade, S.; Bhaskar, V.H. Trapa bispinosa Roxb.: A Review on Nutritional and Pharmacological Aspects. Adv. Pharmacol. Sci. 2014, 2014, 13. [Google Scholar]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant. Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as Immunomodulatory Compounds in the Tumor Microenvironment: Friends or Foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Desprès, P.; El-Kalamouni, C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; González-Aguilar, G.A. Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica cv. Ataulfo) Peels. Int. J. Mol. Sci. 2018, 19, 514. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kaneko, A.; Koseki, J.; Matsubara, Y.; Aiba, S.; Yamasaki, K. Pharmacokinetic Study of Bioactive Flavonoids in the Traditional Japanese Medicine Keigairengyoto Exerting Antibacterial Effects against Staphylococcus aureus. Int. J. Mol. Sci. 2018, 19, 328. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Melchart, D. Therapeutic Effects of Curcumin—From Traditional Past to Present and Future Clinical Applications. Int. J. Mol. Sci. 2019, 20, 3757. [Google Scholar] [CrossRef]
- Toro, M.D.; Nowomiejska, K.; Avitabile, T.; Rejdak, R.; Tripodi, S.; Porta, A.; Reibaldi, M.; Figus, M.; Posarelli, C.; Fiedorowicz, M. Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 3503. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tenover, F.C. Mechanisms of antimicrobial resistance in bacteria. Am. J. Infect. Control. 2006, 34, S3–S10. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Database. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11766372 (accessed on 15 November 2019).
- Fuda, C.; Suvorov, M.; Vakulenko, S.B.; Mobashery, S. The basis for resistance to beta-lactam antibiotics by penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2004, 279, 40802–40806. [Google Scholar] [CrossRef]
- Wang, F.D.; Wu, P.F.; Chen, S.J. Distribution of virulence genes in bacteremic methicillin-resistant Staphylococcus aureus isolates from various sources. J. Microbiol. Immunol. Infect. 2019, 52, 426–432. [Google Scholar] [CrossRef]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol 2018, 9, 2928. [Google Scholar] [CrossRef]
- Joray, M.B.; González, M.L.; Palacios, S.M.; Carpinella, M.C. Antibacterial Activity of the Plant-Derived Compounds 23-Methyl-6-O-desmethylauricepyrone and (Z,Z)-5-(Trideca-4,7-dienyl)resorcinol and Their Synergy with Antibiotics against Methicillin-Susceptible and -Resistant Staphylococcus aureus. J. Agric. Food Chem. 2011, 59, 11534–11542. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2014, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Tagliabue, A.; Rappuoli, R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018, 9, 1068. [Google Scholar] [CrossRef] [PubMed]
- Usman Amin, M.; Khurram, M.; Khan, T.A.; Faidah, H.S.; Ullah Shah, Z.; Ur Rahman, S.; Haseeb, A.; Ilyas, M.; Ullah, N.; Umar Khayam, S.M.; et al. Effects of Luteolin and Quercetin in Combination with Some Conventional Antibiotics against Methicillin-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2016, 17, 1947. [Google Scholar] [CrossRef]
- Chew, Y.L.; Mahadi, A.M.; Wong, K.M.; Goh, J.K. Anti-methicillin-resistance Staphylococcus aureus (MRSA) compounds from Bauhinia kockiana Korth. And their mechanism of antibacterial activity. BMC Complement. Altern. Med. 2018, 18, 70. [Google Scholar] [CrossRef]
- Ding, X.; Ouyang, M.-A.; Shen, Y.-S. Evaluation of Anti-MRSA and Xanthine Oxidase Inhibition Activities of Phenolic Constituents from Plumula nelumbinis. J. CHEM-NY 2015, 2015, 6. [Google Scholar]
- Tayel, A.A.; Shaban, S.M.; Moussa, S.H.; Elguindy, N.M.; Diab, A.M.; Mazrou, K.E.; Ghanem, R.A.; El-Sabbagh, S.M. Bioactivity and application of plant seeds’ extracts to fight resistant strains of Staphylococcus aureus. Ann. Agric. Sci. 2018, 63, 47–53. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simoes, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; van Wamel, W.J.B.; Simões, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef]
- Gibbons, S.; Moser, E.; Kaatz, G.W. Catechin gallates inhibit multidrug resistance (MDR) in Staphylococcus aureus. Planta. Med. 2004, 70, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.U.; Garcia, F.P.; Cortez, D.A.; Ueda-Nakamura, T.; Filho, B.P.; Nakamura, C.V. Antifungal effects of Ellagitannin isolated from leaves of Ocotea odorifera (Lauraceae). Antonie. Van. Leeuwenhoek. 2011, 99, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Esmat, A. Hepatoprotective and antioxidant effect of ellagitannins and galloyl esters isolated from Melaleuca styphelioides on carbon tetrachloride-induced hepatotoxicity in HepG2 cells. Pharm. Biol. 2016, 54, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Huang, J.X.; Ramu, S.; Butler, M.S.; Cooper, M.A. Ramoplanin at bactericidal concentrations induces bacterial membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 6819–6827. [Google Scholar] [CrossRef] [Green Version]
- Alalaiwe, A.; Wang, P.-W.; Lu, P.-L.; Chen, Y.-P.; Fang, J.-Y.; Yang, S.-C. Synergistic Anti-MRSA Activity of Cationic Nanostructured Lipid Carriers in Combination With Oxacillin for Cutaneous Application. Front. Microbiol. 2018, 9, 1493. [Google Scholar] [CrossRef]
- Uribe-García, A.; Paniagua-Contreras, G.L.; Monroy-Pérez, E.; Bustos-Martínez, J.; Hamdan-Partida, A.; Garzón, J.; Alanís, J.; Quezada, R.; Vaca-Paniagua, F.; Vaca, S. Frequency and expression of genes involved in adhesion and biofilm formation in Staphylococcus aureus strains isolated from periodontal lesions. J. Microbiol. Immunol. Infect. 2019, in press. [Google Scholar]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). EUCAST Definitive Document, E.Def 1.2, May 2000: Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Caspar, Y.; Jeanty, M.; Blu, J.; Burchak, O.; Le Pihive, E.; Maigre, L.; Schneider, D.; Jolivalt, C.; Paris, J.M.; Hequet, A.; et al. Novel synthetic bis-indolic derivatives with antistaphylococcal activity, including against MRSA and VISA strains. J. Antimicrob Chemother. 2015, 70, 1727–1737. [Google Scholar]
- Lin, C.Y.; Wang, W.H.; Chen, S.H.; Chang, Y.W.; Hung, L.C.; Chen, C.Y.; Chen, Y.H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.Y.; Lee, C.H.; Chang, Y.W.; Wang, H.M.; Chen, C.Y.; Chen, Y.H. Pheophytin a Inhibits Inflammation via Suppression of LPS-Induced Nitric Oxide Synthase-2, Prostaglandin E2, and Interleukin-1β of Macrophages. Int. J. Mol. Sci. 2014, 15, 22819–22834. [Google Scholar] [CrossRef] [Green Version]
- Pinsky, B.A.; Samson, D.; Ghafghaichi, L.; Baron, E.J.; Banaei, N. Comparison of real-time PCR and conventional biochemical methods for identification of Staphylococcus lugdunensis. J. Clin. Microbiol. 2009, 47, 3472–3477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazumi, T.; Marshall, S.A.; Wilke, W.W.; Diekema, D.J.; Pfaller, M.A.; Jones, R.N. Comparison of the Vitek Gram-Positive Susceptibility 106 card and the MRSA-screen latex agglutination test for determining oxacillin resistance in clinical bloodstream isolates of Staphylococcus aureus. J. Clin. Microbiol. 2001, 39, 53–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, D.T.; Wasney, G.A.; Nosella, M.; Fong, A.; Strynadka, N.C.J. Structural Insights into Inhibition of Escherichia coli Penicillin-binding Protein 1B. J. Biol. Chem. 2017, 292, 979–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joung, D.K.; Mun, S.H.; Choi, S.H.; Kang, O.H.; Kim, S.B.; Lee, Y.S.; Zhou, T.; Kong, R.; Choi, J.G.; Shin, D.W. Antibacterial activity of oxyresveratrol against methicillin-resistant Staphylococcus aureus and its mechanism. Exp. Ther. Med. 2016, 12, 1579–1584. [Google Scholar] [CrossRef]







| Antibiotics/TGII | MIC of MSSA 1 (μg/mL) | MIC of MRSA 2 (μg/mL) | |||
|---|---|---|---|---|---|
| 19615 | 18631 | 18271 | 33591 | ||
| Oxacillin | 4 | 512 | > 512 | > 512 | > 512 |
| Ampicillin | > 512 | > 512 | > 512 | > 512 | > 512 |
| Erythromycin | 128 | > 512 | > 512 | > 512 | > 512 |
| Kanamycin | > 512 | > 512 | > 512 | > 512 | > 512 |
| Levofloxacin | > 512 | > 512 | > 512 | > 512 | 32 |
| Doxycycline | 128 | > 512 | 256 | > 512 | 64 |
| TGII | 64 | 128 | 128 | 128 | 128 |
| MRSA Strain | MIC of Oxacillin (μg/mL) | FIC | Combination Effect | |
|---|---|---|---|---|
| TGII 1 (−) | TGII (+) | |||
| MRSA1 2 | 512 | 4 | 0.008 | Synergy |
| MRSA2 | 512 | 4 | 0.008 | Synergy |
| MRSA3 | 512 | 4 | 0.008 | Synergy |
| MRSA4 | 512 | 2 | 0.004 | Synergy |
| MRSA5 | 512 | 2 | 0.004 | Synergy |
| MRSA6 | 256 | 2 | 0.008 | Synergy |
| MRSA7 | 256 | 2 | 0.008 | Synergy |
| MRSA8 | 128 | 2 | 0.016 | Synergy |
| MRSA9 | 128 | 2 | 0.016 | Synergy |
| MRSA10 | 64 | 2 | 0.031 | Synergy |
| MRSA11 | 64 | 4 | 0.063 | Additive |
| MRSA12 | 64 | 4 | 0.063 | Additive |
| MRSA13 | 64 | 2 | 0.031 | Synergy |
| MRSA14 | 32 | 2 | 0.063 | Additive |
| Antibiotics | TGII 1 | MSSA 2 | MRSA 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 19615 | 18631 | 18271 | 33591 | ||||||||
| MIC 3 | FIC | MIC | FIC | MIC | FIC | MIC | FIC | MIC | FIC | ||
| Oxacillin | − | 4 | − | 512 | − | 512 | − | 512 | − | 512 | − |
| + | 2 | 0.5 | 4 | 0.008 | 8 | 0.02 | 16 | 0.03 | 2 | 0.004 | |
| Ampicillin | − | 512 | − | 512 | − | 512 | − | 512 | − | 512 | − |
| + | 256 | 0.50 | 512 | 1.00 | 512 | 1.00 | 16 | 0.031 | 512 | 1.00 | |
| Vancomycin | − | 32 | − | 16 | − | 16 | − | 16 | − | 4 | − |
| + | 32 | 1.00 | 32 | 2.00 | 32 | 2.00 | 32 | 2.000 | 4 | 1.00 | |
| Levofloxacin | − | 512 | − | 512 | − | 256 | − | 512 | − | 512 | − |
| + | 512 | 1.00 | 512 | 1.00 | 512 | 2.00 | 512 | 1.00 | 512 | 1.00 | |
| Erythromycin | − | 128 | − | 512 | − | 512 | − | 512 | − | 512 | − |
| + | 64 | 0.50 | 512 | 1.00 | 512 | 1.00 | 512 | 1.00 | 512 | 1.00 | |
| Kanamycin | − | 512 | − | 512 | − | 512 | − | 512 | − | 512 | − |
| + | 512 | 1.00 | 512 | 1.00 | 512 | 1.00 | 512 | 1.00 | 512 | 1.00 | |
| Doxycycline | − | 128 | − | 512 | − | 256 | − | 512 | − | 64 | − |
| + | 8 | 0.063 | 8 | 0.016 | 2 | 0.008 | 2 | 0.004 | 64 | 1.00 | |
| Target Gene | Product Length (bp) | Sequence | |
|---|---|---|---|
| mecA | 94 | Forward | CTGCTATCCACCCTCAAACAG |
| Reverse | TCTTCGTTACTCATGCCATACA | ||
| ftsZ (S. aureus) | 217 | Forward | TTACTGGTGGCGAGTCATTG |
| Reverse | TTTACGCTTGTTCCGAATCC | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-W.; Huang, W.-C.; Lin, C.-Y.; Wang, W.-H.; Hung, L.-C.; Chen, Y.-H. Tellimagrandin II, A Type of Plant Polyphenol Extracted from Trapa bispinosa Inhibits Antibiotic Resistance of Drug-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2019, 20, 5790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225790
Chang Y-W, Huang W-C, Lin C-Y, Wang W-H, Hung L-C, Chen Y-H. Tellimagrandin II, A Type of Plant Polyphenol Extracted from Trapa bispinosa Inhibits Antibiotic Resistance of Drug-Resistant Staphylococcus aureus. International Journal of Molecular Sciences. 2019; 20(22):5790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225790
Chicago/Turabian StyleChang, Yu-Wei, Wan-Chun Huang, Chun-Yu Lin, Wen-Hung Wang, Ling-Chien Hung, and Yen-Hsu Chen. 2019. "Tellimagrandin II, A Type of Plant Polyphenol Extracted from Trapa bispinosa Inhibits Antibiotic Resistance of Drug-Resistant Staphylococcus aureus" International Journal of Molecular Sciences 20, no. 22: 5790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225790