Predictive Value of Serum Antibodies and Point Mutations of AQP4, AQP1 and MOG in A Cohort of Spanish Patients with Neuromyelitis Optica Spectrum Disorders
Abstract
:1. Introduction
2. Results
2.1. Determination of Antibodies against AQP4, MOG, and AQP1
2.2. Comparison of CBA Results for Anti-AQP4 Antibody Determinations
2.3. Comparison of CBA and ELISA Results for Anti-AQP4 Antibody Determinations
2.4. Analysis by CBA and ELISA of Anti-MOG Antibodies
2.5. Analysis by CBA and ELISA of Anti-AQP1 Antibodies
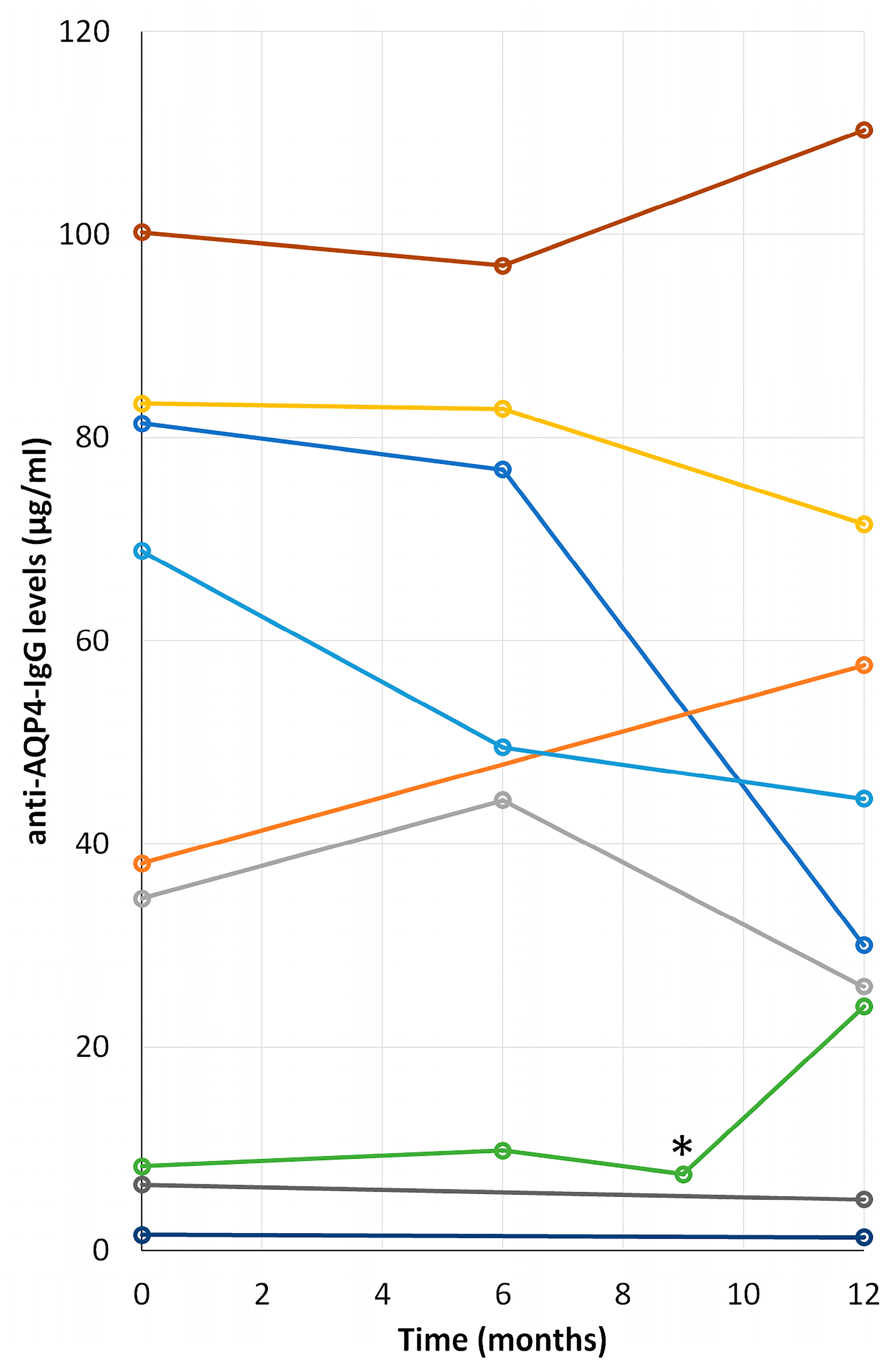
2.6. Time Course Progression of Anti-AQP4 IgG Levels in NMOsd(+) Patients
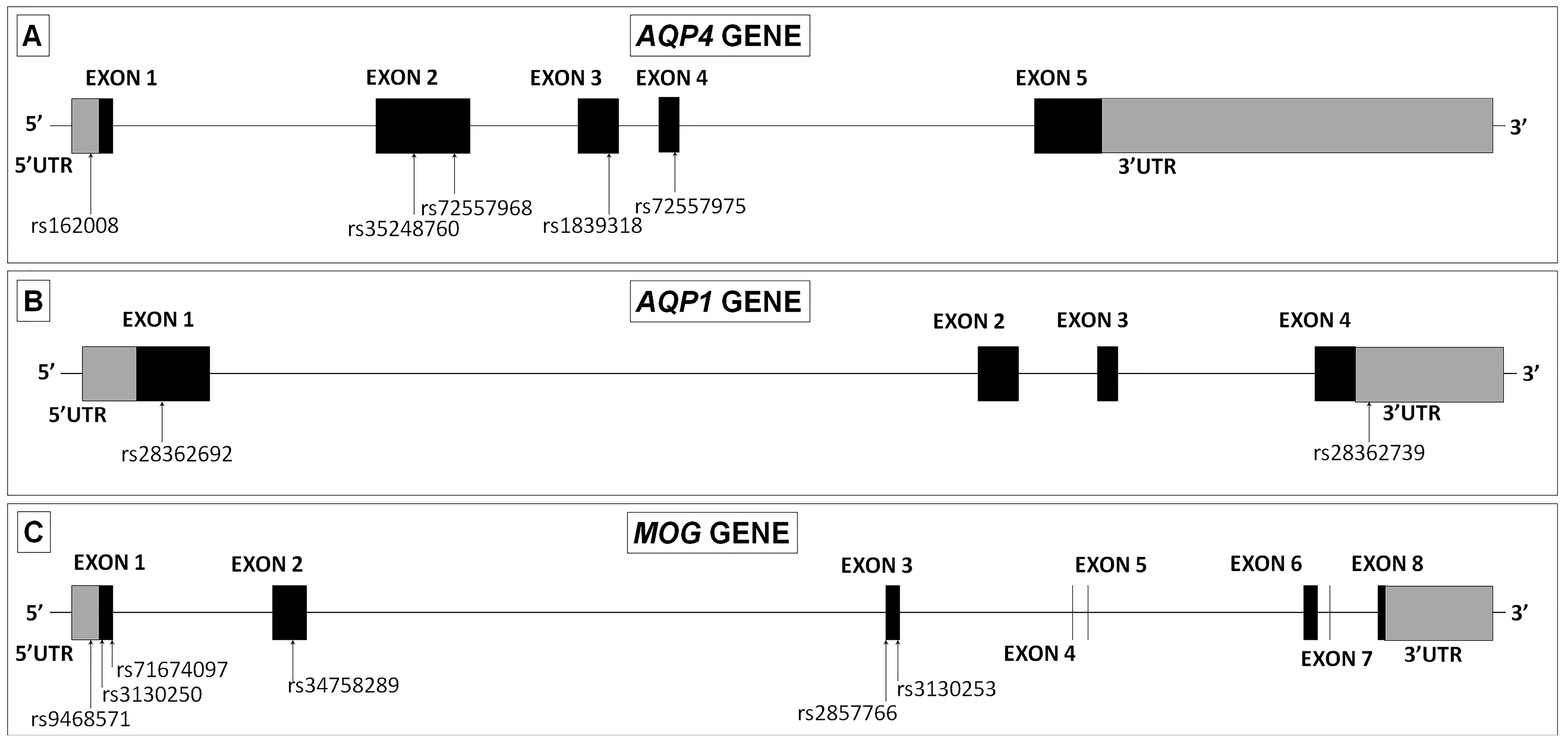
2.7. Genomic DNA Analysis of Variants in AQP4, AQP1 and MOG Genes
3. Discussion
4. Materials and Methods
4.1. Subjects and Serum Collection
4.2. Immunofluorescence Assay
4.2.1. Immunoassay Developed in Our Laboratory
4.2.2. Euroimmun® Assay
4.3. ELISA for Anti-AQP4, Anti-MOG, and Anti-AQP1 Antibodies
4.3.1. ELISA for Anti-AQP4 or Anti-MOG IgG
4.3.2. ELISA for Anti-AQP1
4.4. Analysis of AQP4, AQP1 and MOG Genes
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NMO | Neuromyelitis optica |
| NMOsd | NMO spectrum disorder |
| NMOsd(+) | NMOsd patients that present antibodies against AQP4 |
| NMOsd(–) | NMOsd patients that do not present antibodies against AQP4 |
| CBA | Cell-based assays |
| ELISA | Enzyme-Linked ImmunoSorbent Assay |
| AQP4 | Aquaporin-4 |
| AQP1 | Aquaporin-1 |
| MOG | Myelin oligodendrocyte glycoprotein |
References
- Uzawa, A.; Mori, M.; Kuwabara, S. Neuromyelitis optica: Concept, immunology and treatment. J. Clin. Neurosci. 2014, 21, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Wingerchuk, D.M.; Kryzer, T.J.; Pittock, S.J.; Lucchinetti, C.F.; Fujihara, K.; Nakashima, I.; Weinshenker, B. A serum autoantibody marker of neuromyelitis optica. Lancet 2004, 364, 2106–2112. [Google Scholar] [CrossRef]
- Makhani, N.; Bigi, S.; Banwell, B.; Shroff, M. Diagnosing neuromyelitis optica. Neuroimaging Clin. N. Am. 2013, 23, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Phuan, P.W.; Asavapanumas, N.; Tradtrantip, L. Biology of AQP4 and Anti-AQP4 antibody: Therapeutic implications for NmO. Brain Pathol. 2013, 23, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, B.G.; Wingerchuk, D.M. Neuromyelitis optica: Clinical syndrome and the NMO-IgG autoantibody marker. Curr. Top. Microbiol. Immunol. 2008, 318, 343–356. [Google Scholar] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef]
- Katz Sand, I. Neuromyelitis optica spectrum disorders. Continuum 2016, 22, 864–896. [Google Scholar]
- Melamed, E.; Levy, M.; Waters, P.J.; Sato, D.K.; Bennett, J.L.; John, G.R.; Hooper, D.C.; Saiz, A.; Bar-Or, A.; Kim, H.J.; et al. Update on biomarkers in neuromyelitis optica. Neurol. Neuroimmunol. Neuro. 2015, 2, e134. [Google Scholar] [CrossRef]
- Xu, M.; Xiao, M.; Li, S.; Yang, B. Aquaporins in nervous system. Adv. Exp. Med. Biol. (Springer Netherlands) 2017, 969, 81–103. [Google Scholar]
- Tzartos, J.S.; Stergiou, C.; Kilidireas, K.; Zisimopoulou, P.; Thomaidis, T.; Tzartos, S.J. Anti-aquaporin-1 autoantibodies in patients with neuromyelitis optica spectrum disorders. PLoS ONE 2013, 8, e74773. [Google Scholar] [CrossRef]
- Long, Y.; Zheng, Y.; Shan, F.; Chen, M.; Fan, Y.; Zhang, B.; Gao, C.; Gao, Q.; Yang, N. Development of a cell-based assay for the detection of anti-aquaporin 1 antibodies in neuromyelitis optica spectrum disorders. J. Neuroimmunol. 2014, 273, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, E.; Tzartos, J.; Ekizoglu, E.; Stergiou, C.; Zisimopoulou, P.; Coban, A.; Shugaiv, E.; Turkoglu, R.; Kurtuncu, M.; Baykan, B.; et al. Aquaporin-1 antibody in neuromyelitis optical patients. Eur. Neurol. 2014, 72, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Kitley, J.; Woodhall, M.; Waters, P.; Leite, M.I.; Devenney, E.; Craig, J.; Palace, J.; Vincent, A. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology 2012, 79, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Reindl, M.; Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 2019, 15, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Reindl, M.; Di Pauli, F.; Rostásy, K.; Berger, T. The spectrum of MOG autoantibody-associated demyelinating diseases. Nat. Rev. Neurol. 2013, 9, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, S.; Mazzi, B.; Tassara, M.; Colombo, B.; Guerrieri, S.; Giordano, A.; Preziosa, P.; Comola, M.; Esposito, F.; Martinelli, V.; et al. Neuromyelitis optica spectrum disorder and multiple sclerosis in a Sardinian family. Mult. Scler. Relat. Disord. 2018, 25, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Tsai, M.H.; Lien, C.Y.; Huang, Y.J.; Chang, W.N. Intra-family phenotype variations in familial neuromyelitis optica spectrum disorders. Mult. Scler. Relat. Disord. 2019, 30, 57–62. [Google Scholar] [CrossRef]
- Wu, Y.; Zhong, L.; Geng, J. Neuromyelitis optica spectrum disorder: Pathogenesis, treatment, and experimental models. Mult. Scler. Relat. Disord. 2019, 27, 412–418. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Xiao, H.-Q.; Chen, H.-X.; Liu, Y.-P.; Ding, X.-D. The single nucleotide polymorphism site of aquaporin-4 gene in patients with neuromyelitis optica. Exp. Ther. Med. 2017, 14, 6017–6021. [Google Scholar] [CrossRef]
- Ogasawara, M.; Meguro, A.; Sakai, T.; Mizuki, N.; Takahashi, T.; Fujihara, K.; Tsuneoka, H.; Shikishima, K. Genetic analysis of the aquaporin-4 gene for anti-AQP4 antibody-positive neuromyelitis optica in a Japanese population. Jpn. J. Ophthalmol. 2016, 7, 76–83. [Google Scholar] [CrossRef]
- Yang, T.-T.; He, Y.; Xiang, Y.-J.; Ao, D.-H.; Wang, Y.-Y.; Zhang, Q.; He, X.-J.; Zhong, S.-S.; Wu, J.; Liu, G.-Z. No association of AQP4 polymorphisms with neuromyelitis optica and multiple sclerosis. Transl. Neurosci. 2016, 7, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Matiello, M.; Schaefer-Klein, J.L.; Hebrink, D.D.; Kingsbury, D.J.; Atkinson, E.J.; Weinshenker, B.G. Genetic analysis of aquaporin-4 in neuromyelitis optica. Neurology 2011, 77, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Sánchez Gomar, I.; Díaz Sánchez, M.; Uclés Sánchez, A.J.; Casado Chocán, J.L.; Ramírez-Lorca, R.; Serna, A.; Villadiego, J.; Toledo-Aral, J.J.; Echevarría, M. An immunoassay that distinguishes real neuromyelitis optica signals from a labeling detected in patients receiving natalizumab. BMC Neurol. 2014, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Gomar, I.; Diaz Sanchez, M.; Ucles Sanchez, A.J.; Casado Chocan, J.L.; Suarez-Luna, N.; Ramirez-Lorca, R.; Villadiego, J.; Toledo-Aral, J.J.; Echevarria, M. Comparative analysis for the presence of IgG Anti-aquaporin-1 in patients with NMO-spectrum disorders. Int. J. Mol. Sci. 2016, 17, e1195. [Google Scholar] [CrossRef]
- Kim, S.M.; Kim, S.J.; Lee, H.J.; Kuroda, H.; Palace, J.; Fujihara, K. Differential diagnosis of neuromyelitis optica spectrum disorders. Ther. Adv. Neurol. Disord. 2017, 10, 265–289. [Google Scholar] [CrossRef]
- Waters, P.J.; McKeon, A.; Leite, M.I.; Rajasekharan, S.; Lennon, V.A.; Villalobos, A.; Palace, J.; Mandrekar, J.N.; Vincent, A.; Bar-Or, A.; et al. Serologic diagnosis of NMO: A multicenter comparison of aquaporin-4-IgG assays. Neurology 2012, 78, 665–671. [Google Scholar] [CrossRef]
- Schanda, K.; Waters, P.; Holzer, H.; Aboulenein-Djamshidian, F.; Leite, M.I.; Palace, J.; Vukusic, S.; Marignier, R.; Berger, T.; Reindl, M. Antibodies to aquaporin-1 are not present in neuromyelitis optica. Neurol. Neuroimmunol. Neuroinflammation 2015, 2, e160. [Google Scholar] [CrossRef]
- Gomez-Lira, M.; Moretto, G.; Bonamini, D.; Benedetti, M.D.; Pignatti, P.F.; Rizzuto, N.; Salviati, A. Myelin oligodendrocyte glycoprotein polymorphisms and multiple sclerosis. J. Neuroimmunol. 2002, 133, 241–243. [Google Scholar] [CrossRef]
- D’Alfonso, S.; Bolognesi, E.; Guerini, F.R.; Barizzone, N.; Bocca, S.; Ferrante, D.; Castelli, L.; Bergamaschi, L.; Agliardi, C.; Ferrante, P.; et al. A sequence variation in the MOG gene is involved in multiple sclerosis susceptibility in Italy. Genes Immun. 2008, 9, 7–15. [Google Scholar] [CrossRef]
- Sorani, M.D.; Zado, Z.; Hurowitz, E.; Yan, D.; Giacomini, K.M.; Manley, G.T. Novel variants in human Aquaporin-4 reduce cellular water permeability. Hum. Mol. Genet. 2008, 17, 2379–2389. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Diagnosis | Number of Patients | Gender (Female/Male) | Mean Age at Inclusion ± SD | AQP4 | MOG | AQP1 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CBA | EURO INMUN | ELISA | EURO INMUN | ELISA | BCA | ELISA | ||||
| 1. NMOsd | 18 | 15/3 | 56 ± 13 | 11 | 12 | 7 | - | - | - | - |
| 2. MS | 48 | 32/16 | 44 ± 12 | - | - | - | - | - | - | - |
| 2.1. RRMS | 37 | 28/9 | 40 ± 10 | - | - | - | - | - | - | - |
| 2.2. PPMS | 7 | 2/5 | 62 ± 12 | - | - | - | - | - | - | - |
| 2.3. SPMS | 4 | 2/2 | 47 ± 11 | - | - | - | - | - | - | - |
| 3. Idiopathic | 14 | 9/6 | 47 ± 10 | - | - | - | - | - | - | - |
| 3.1. Isolated episode | 3 | 3/0 | 34 ± 18 | - | - | - | - | - | - | - |
| 3.2. Recurrent idiopathic ON | 11 | 6/5 | 49 ± 12 | - | - | - | - | - | - | - |
| 4. Idiopathic myelitis | 22 | 14/8 | 49 ± 13 | - | - | - | - | - | - | - |
| 4.1. Isolated episode | 17 | 9/8 | 51 ± 14 | - | - | - | - | - | - | - |
| 4.1.1. < 3 vertebral segments | 5 | 2/3 | 40 ± 5 | - | - | - | - | - | - | - |
| 4.1.2. > 3 vertebral segments | 12 | 7/5 | 55 ± 14 | - | - | - | - | - | - | - |
| 4.2. Recurrent idiopathic myelitis | 5 | 5/0 | 42 ± 9 | - | - | - | 1 | - | - | - |
| 5. Brainstem clinically isolated syndrome | 4 | 3/1 | 43 ± 19 | - | - | - | - | - | - | - |
| 6. Other pathologies | 5 | 3/2 | 42 ± 16 | - | - | - | - | - | - | - |
| 6.1. Glaucoma with visual défic | 8 | 1/0 | 52 ± 10 | - | - | - | - | - | - | - |
| 6.2. Spinal tumor | 1 | 0/1 | 39 ± 0 | - | - | - | - | - | - | - |
| 6.3. Behcet disease | 1 | 1/0 | 36 ± 0 | - | - | - | - | - | - | - |
| 6.4. Leber optic neuropathy | 1 | 1/0 | 21 ± 0 | - | - | - | - | - | - | - |
| 6.5. Ischemic optic neuropathy | 1 | 0/1 | 62 ± 0 | - | - | - | - | - | - | - |
| 7. Healthy controls | 8 | 5/3 | 36 ± 11 | - | - | - | - | - | - | - |
| GENE | SNP | Change | Amino Acid Codon | Amino Acid Change | NMOSD(+) N = 12 | NMOSD(–) N = 6 | MS N = 16 |
|---|---|---|---|---|---|---|---|
| AQP4 | rs162008 | c.-39G > A | - | - | 5 | 0 | 7 |
| rs35248760 | c.201G > T | [CCG] > [CCT] | Pro67Pro | 4 | 4 | 5 | |
| rs72557968 | c.366G > A | [CAG] > [CAA] | Gln122Gln | 1 | 0 | 0 | |
| rs1839318 | c.492G > A | [TTG] > [TTA] | Leu164Leu | 1 | 0 | 0 | |
| rs72557975 | c.671T > C | [ATG] > [ACG] | Met224Thr | 1 | 0 | 0 | |
| AQP1 | rs28362692 | c.134C > T | [GCG] > [GTG] | Ala45Val | 0 | 1 | 0 |
| rs28362739 | dupA(G)4 | - | - | 4 | 1 | 1 | |
| MOG | rs9468571 | c.-93T > C | - | - | 1 | 0 | 2 |
| rs3130250 | c.15A > G | [TCA] > [TCG] | Ser5Ser | 2 | 0 | 4 | |
| rs71674097 | c.47_49TCC | [CTCC] > [CAA] | Leu22del | 1 | 1 | 3 | |
| rs34758289 | c.306A > G | [AAA] > [AAG] | Lys102Lys | 1 | 0 | 1 | |
| rs2857766 | c.511G > C | [GTT] > [CTT] | Val171Leu | 5 | 3 | 1 | |
| rs3130253 | c.520G > A | [GTC] > [ATC] | Val174Ile | 2 | 0 | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Miranda, P.; Morón-Civanto, F.J.; Martínez-Olivo, M.d.M.; Suárez-Luna, N.; Ramírez-Lorca, R.; Lebrato-Hernández, L.; Lamas-Pérez, R.; Navarro, G.; Abril-Jaramillo, J.; García-Sánchez, M.I.; et al. Predictive Value of Serum Antibodies and Point Mutations of AQP4, AQP1 and MOG in A Cohort of Spanish Patients with Neuromyelitis Optica Spectrum Disorders. Int. J. Mol. Sci. 2019, 20, 5810. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225810
García-Miranda P, Morón-Civanto FJ, Martínez-Olivo MdM, Suárez-Luna N, Ramírez-Lorca R, Lebrato-Hernández L, Lamas-Pérez R, Navarro G, Abril-Jaramillo J, García-Sánchez MI, et al. Predictive Value of Serum Antibodies and Point Mutations of AQP4, AQP1 and MOG in A Cohort of Spanish Patients with Neuromyelitis Optica Spectrum Disorders. International Journal of Molecular Sciences. 2019; 20(22):5810. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225810
Chicago/Turabian StyleGarcía-Miranda, Pablo, Francisco J. Morón-Civanto, Maria del Mar Martínez-Olivo, Nela Suárez-Luna, Reposo Ramírez-Lorca, Lucía Lebrato-Hernández, Raquel Lamas-Pérez, Guillermo Navarro, Javier Abril-Jaramillo, Maria Isabel García-Sánchez, and et al. 2019. "Predictive Value of Serum Antibodies and Point Mutations of AQP4, AQP1 and MOG in A Cohort of Spanish Patients with Neuromyelitis Optica Spectrum Disorders" International Journal of Molecular Sciences 20, no. 22: 5810. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225810







