In Vitro Effects of Vaspin on Porcine Granulosa Cell Proliferation, Cell Cycle Progression, and Apoptosis by Activation of GRP78 Receptor and Several Kinase Signaling Pathways Including MAP3/1, AKT, and STAT3
Abstract
:1. Introduction
2. Results
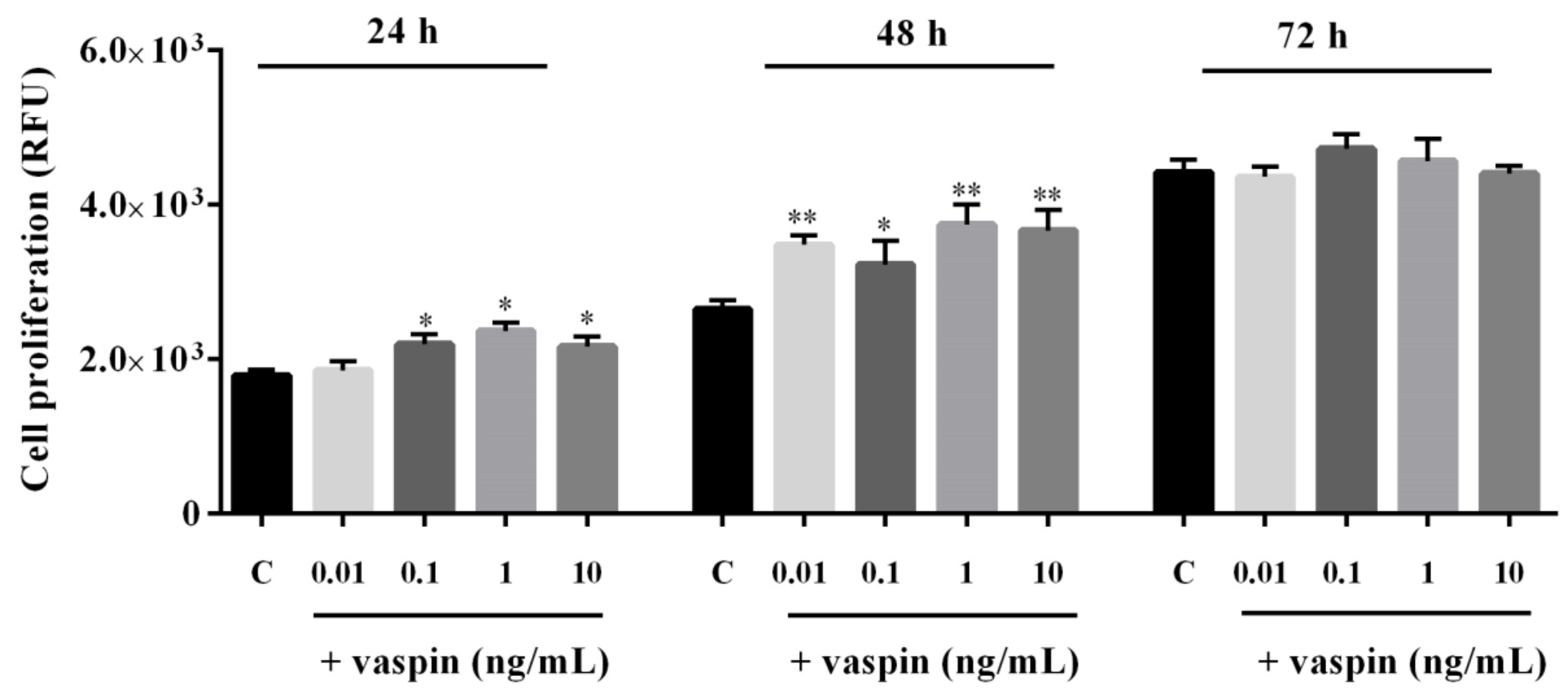
2.1. Dose- and Time-Dependent Effects of Vaspin on Gc Proliferation
2.2. Effect of Vaspin on Gc Cycle Regulation
2.3. Involvement of the GRP78 Receptor and MAP3/1, AKT, STAT3, and PRKAA1 Kinases in Proliferative Effect of Vaspin on Gc Proliferation
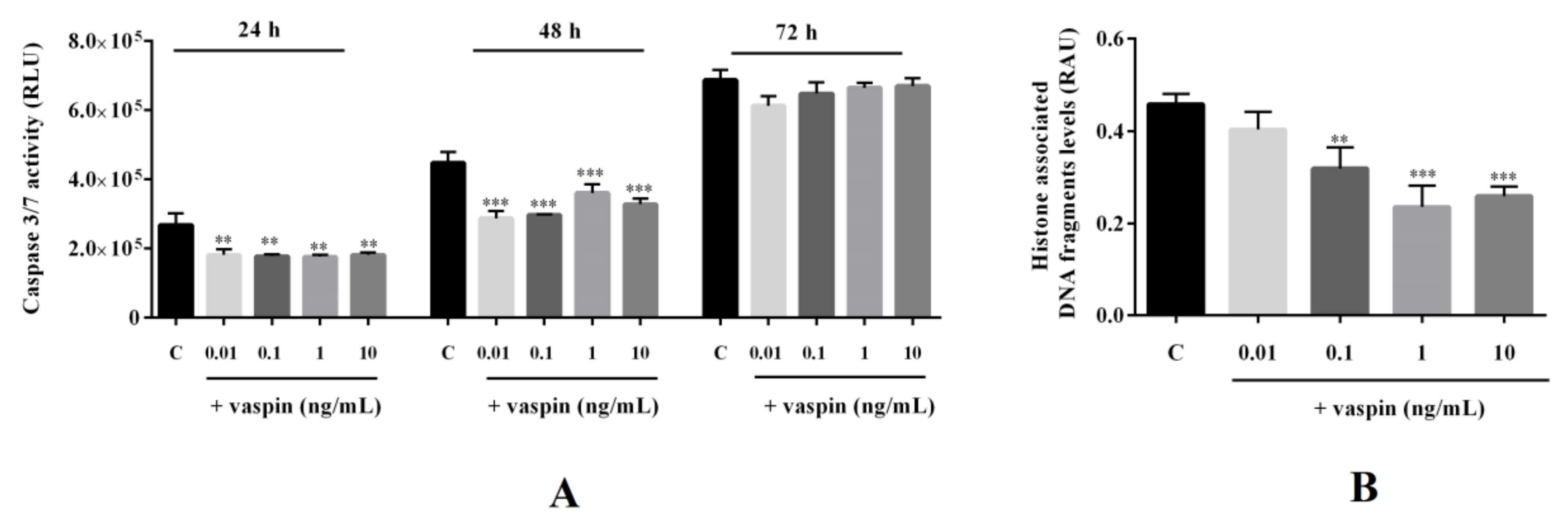
2.4. Effect of Vaspin on Caspase-3 and -7 Activity and Histone-Associated DNA Fragment Levels in Gc
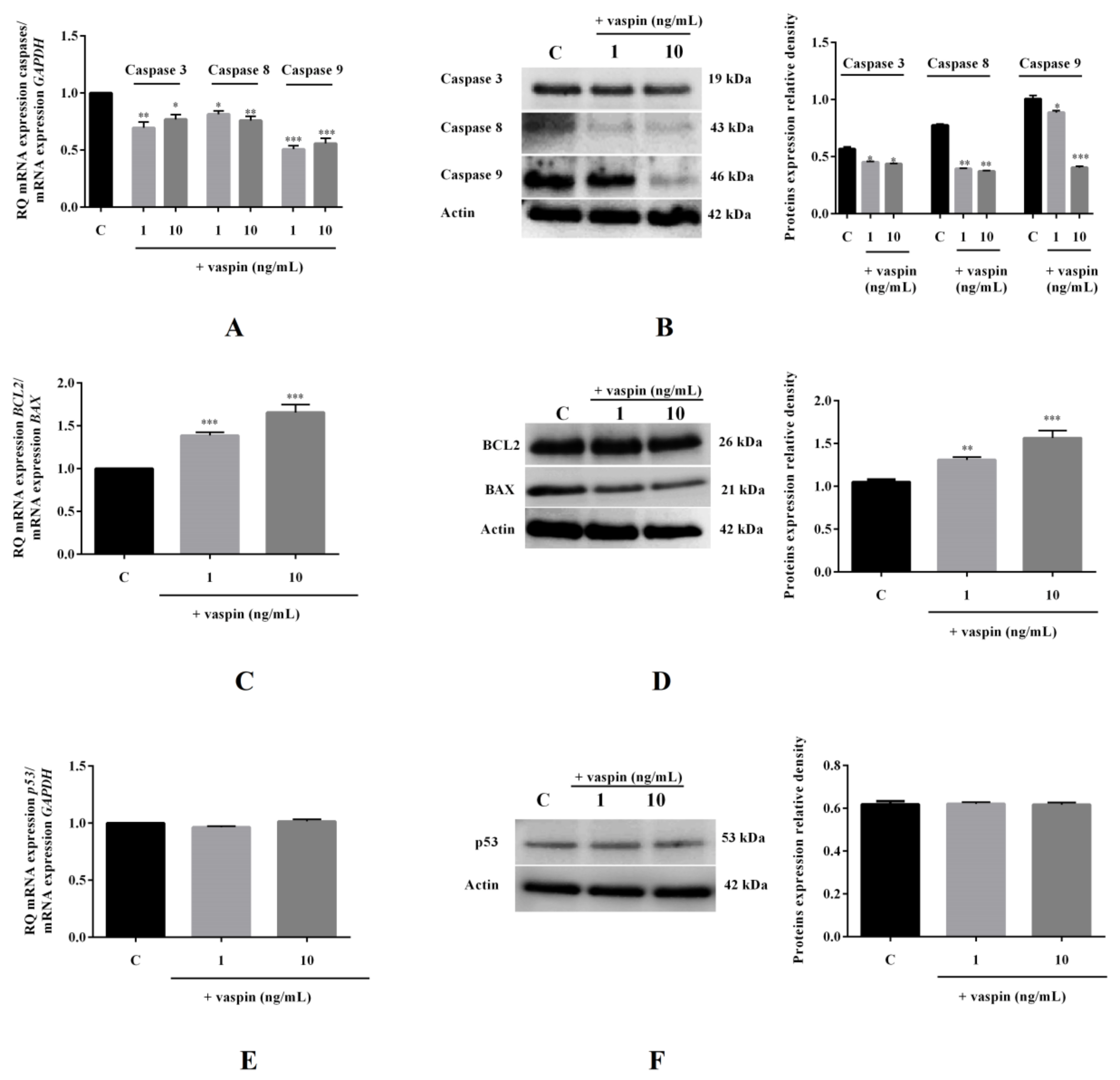
2.5. Effect of Vaspin on mRNA and Protein Expression of Caspases-3,-8, and -9; BAX; BCL2; and p53 in Gc
2.6. Involvement of the GRP78 Receptor and MAP3/1, AKT, STAT3, and PRKAA1 Kinases in the Antiapoptotic Effect of Vaspin in Gc
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Gc In Vitro Cultures
4.2.1. Dose- and Time-Dependent Effects of Vaspin on Cell Proliferation and Caspase-3/7 Activity
4.2.2. Effect of Vaspin on Levels of Histone-Associated DNA Fragments and the Regulation of the Cell Cycle
4.2.3. Effect of Vaspin on Cyclin Protein Expression as Well Caspases and BAX/BCL2 mRNA and Protein Expression
4.2.4. Assessing the Involvement of the GRP78 Receptor in Vaspin-Mediated Gc Proliferation and Caspase-3/7 Activity
4.2.5. Involvement of MAP3/1, AKT, STAT3, and PRKAA1 Kinases in Vaspin Induction of Proliferation and Caspase-3/7 Activity
4.3. Gene Silencing
4.4. AlamarBlue Assay
4.5. Flow Cytometry
4.6. Caspase-Glo 3/7 Assay
4.7. Cell-Death Detection ELISA Kit
4.8. Real-Time PCR
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Gc | Granulosa cells |
| BCL2 | B-cell lymphoma |
| MAP3/1/ERK1/2 | Mitogen-activated kinase |
| STAT3 | Janus kinase |
| AKT | Protein kinase B |
| AMPK/PRKAA1 | 5′ AMP-activated protein kinase |
| COC | Cumulus cell–oocyte complex |
| OLETF | Otsuka Long-Evans Tokushima fatty |
| P4 | Progesterone |
| GRP78 | Binding immunoglobulin protein |
| TNF-α | Tumor necrosis factor |
| IGF1 | Insulin like growth factor type 1 |
| RFU | Relative Fluorescence Unit |
| RLU | Relative Luminescence Unit |
| RAU | Relative Absorbance Unit |
| FSH | Folliculotropin |
| PCNA | Proliferation-related peptide |
| LH | Luteinizing hormone |
| FBS | Foetal bovine serum |
| PBS | Phosphate buffered saline |
| Ct | Cycle threshold number |
| HRP | Horseradish peroxidase |
| BSA | Bovine serum albumin |
| E2 | Estradiol |
References
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Duronio, R.J.; Xiong, Y. Signaling pathways that control cell proliferation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008904. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Roychoudhury, S.; Slivkova, J.; Sirotkin, A.; Capcarova, M.; Massanyi, P. In vitro study on the effects of lead and mercury on porcine ovarian granulosa cells. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 320–321. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Capcarova, M.; Sirotkin, A.; Medvedova, M.; Kovacik, J. Cobalt-induced changes in the IGF-I and progesterone release, expression of proliferation- and apoptosis-related peptides in porcine ovarian granulosa cells in vitro. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2010, 45, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Tománek, M.; Chronowska, E. Immunohistochemical localization of proliferating cell nuclear antigen (PCNA) in the pig ovary. Folia Histochem. Cytobiol. 2006, 44, 269–274. [Google Scholar] [PubMed]
- Hussein, M.R. Apoptosis in the ovary: Molecular mechanisms. Hum. Reprod. Update 2005, 11, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Ouyang, G.; Bao, S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell Mol. Med. 2005, 9, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Liu, Y.X.; Liu, X.; Wang, S.L.; Li, P.; Lin, X.H.; Sui, C.L.; Xu, C.; Qi, B.; Tong, Q. Effects of Gui Zhu Yi Kun formula on the P53/AMPK pathway of autophagy in granulosa cells of rats with polycystic ovary syndrome. Exp. Ther. Med. 2017, 13, 3567–3573. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Reverchon, M.; Cloix, L.; Froment, P.; Ramé, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2012, 56, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.Y.; Liu, Z.; Shimada, M.; Sterneck, E.; Johnson, P.F.; Hedrick, S.M.; Richards, J.S. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009, 324, 938–941. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Shimada, M. The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process. J. Reprod. Dev. 2012, 58, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Drwal, E.; Wróbel, A.; Gregoraszczuk, E.Ł. Resistin is a survival factor for porcine ovarian follicular cells. Reproduction 2015, 150, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Shikata, K. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, R.; Moriya, J.; Yamakawa, J.; Sumino, H.; Kanda, T.; Takahashi, T. A novel adipocytokine, visceral adipose tissue-derived serine protease inhibitor (vaspin), and obesity. J. Int. Med. Res. 2008, 36, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Blüher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhang, C.; Wang, J.; Wang, C.; Lan, X.; Zhang, C.; Lei, C.; Chen, H. mRNA expression pattern and association study with growth traits of bovine vaspin gene. Mol. Biol. Rep. 2013, 40, 4499–4505. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Barbe, A.; Staub, C.; Gregoraszczuk, E.; Dupont, J.; Rak, A. Vaspin in the pig ovarian follicles: Expression and regulation by different hormones. Reproduction 2019, 158, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Heiker, J.T. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. J. Pept. Sci. 2014, 20, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Visceral adipose tissue-derived serine proteinase inhibitor inhibits apoptosis of endothelial cells as a ligand for the cell-surface GRP78/voltage-dependent anion channel complex. Circ. Res. 2013, 112, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wey, S.; Zhang, Y.; Ye, R.; Lee, A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal. 2009, 11, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Hao, Y.; Li, B.; Zou, J.; Li, X.; Wei, C.; Liu, F.; Zhang, Z. Vaspin Prevents Tumor Necrosis Factor-α-Induced Apoptosis in Cardiomyocytes by Promoting Autophagy. J. Cardiovasc. Pharmacol. 2018, 77, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhuang, J.; Li, H.; Zhu, G.; Zhou, S.; Li, W.; Peng, W.; Xu, Y. Vaspin attenuates the progression of atherosclerosis by inhibiting ER stress-induced macrophage apoptosis in apoE-/- mice. Mol. Med. Rep. 2016, 13, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Jiang, Y.; Shan, P.F.; Shen, J.; Liang, Q.H.; Cui, R.R.; Liu, Y.; Liu, G.Y.; Wu, S.S.; Lu, Q.; et al. Vaspin attenuates the apoptosis of human osteoblasts through ERK signaling pathway. Amino. Acids 2013, 44, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Wang, D.; Zhang, C.; Tang, X.; He, J.; Zhao, Y.; Deng, W.; Deng, X. Vaspin protects against LPS-induced ARDS by inhibiting inflammation, apoptosis and reactive oxygen species generation in pulmonary endothelial cells via the Akt/GSK-3β pathway. Int. J. Mol. Med. 2017, 40, 1803–1817. [Google Scholar] [CrossRef] [PubMed]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 9, 4431. [Google Scholar] [CrossRef] [PubMed]
- Bongrani, A.; Mellouk, N.; Rame, C.; Cornuau, M.; Guérif, F.; Froment, P.; Dupont, J. Ovarian Expression of Adipokines in Polycystic Ovary Syndrome: A Role for Chemerin, Omentin, and Apelin in Follicular Growth Arrest and Ovulatory Dysfunction. Int. J. Mol. Sci. 2019, 20, 3778. [Google Scholar] [CrossRef] [PubMed]
- Messini, C.I.; Vasilaki, A.; Korona, E.; Anifandis, G.; Georgoulias, P.; Dafopoulos, K.; Garas, A.; Daponte, A.; Messinis, I.E. Effect of resistin on estradiol and progesterone secretion from human luteinized granulosa cells in culture. Syst. Biol. Reprod. Med. 2019, 65, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Cao, Z.; Gai, Y.; Liu, M.; Gao, M.; Chen, M.; Ning, Z.; Luan, X. Effects of recombinant goose adiponectin on steroid hormone secretion in Huoyan geese ovarian granulosa cells. Anim. Reprod. Sci. 2019, 205, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczynska, E.; Dawid, M.; Dupont, J.; Rak, A. Regulatory role of vaspin in porcine ovarian follicles: Effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase a pathway. In vitro study. Biol. Reprod. 2019, in press. [Google Scholar]
- Phalitakul, S.; Okada, M.; Hara, Y.; Yamawaki, H. Vaspin prevents methylglyoxal-induced apoptosis in human vascular endothelial cells by inhibiting reactive oxygen species generation. Acta Physiol. (Oxf.) 2013, 209, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Emery, B.R.; Carrell, D.T. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol. Reprod. 2001, 64, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auguet, T.; Quintero, Y.; Riesco, D.; Morancho, B.; Terra, X.; Crescenti, A.; Broch, M.; Aguilar, C.; Olona, M.; Porras, J.A.; et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med. Genet. 2011, 12, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirotkin, A.V.; Florkovičová Koničková, I.; Schaeffer, H.J.; Laurincik, J.; Harrath, A.H. Interrelationships between ovarian follicles grown in culture and possible mediators. Reprod. Biol. 2017, 17, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, C.; Skotheim, J.M.; De Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, I.; Dynlacht, B.D. New insights into cyclins, CDKs, and cell cycle control. Semin. Cell Dev. Biol. 2005, 16, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Kolesarova, A.; Capcarova, M.; Sirotkin, A.V.; Medvedova, M.; Kovacik, J. In vitro assessment of silver effect on porcine ovarian granulosa cells. J. Trace Elem. Med. Biol. 2011, 25, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.H.; Wang, L.; Riaz, H.; Wu, J.B.; Yuan, Y.F.; Han, L.; Wang, Y.L.; Zhao, Y.; Dan, Y.; Huo, L.J. Knockdown of CEBPβ by RNAi in porcine granulosa cells resulted in S phase cell cycle arrest and decreased progesterone and estradiol synthesis. J. Steroid. Biochem. Mol. Biol. 2014, 143, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xia, G.; Tsang, B.K. Regulation of cyclin D2 expression and degradation by follicle-stimulating hormone during rat granulosa cell proliferation in vitro. Biol. Reprod. 2013, 88, 57. [Google Scholar] [CrossRef] [PubMed]
- Spicer, L.J.; Chamberlain, C.S.; Francisco, C.C. Ovarian action of leptin: Effects on insulin-like growth factor-I-stimulated function of granulosa and thecal cells. Endocrine 2000, 12, 53–59. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Grossmann, R. Leptin directly controls proliferation, apoptosis and secretory activity of cultured chicken ovarian cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Mlyncek, M.; Makarevich, A.V.; Florkovicová, I.; Hetényi, L. Leptin affects proliferation, apoptosis- and protein kinase A-related peptides in human ovarian granulosa cells. Physiol. Res. 2008, 57, 437–442. [Google Scholar] [PubMed]
- Maillard, V.; Froment, P.; Ramé, C.; Uzbekova, S.; Elis, S.; Dupont, J. Expression and effect of resistin on bovine and rat granulosa cell steroidogenesis and proliferation. Reproduction 2011, 141, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirotkin, A.V.; Meszarosová, M. Comparison of effects of leptin and ghrelin on porcine ovarian granulosa cells. Domest. Anim. Endocrinol. 2010, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Drwal, E.; Rame, C.; Knapczyk-Stwora, K.; Słomczyńska, M.; Dupont, J.; Gregoraszczuk, E.L. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017, 96, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Mellouk, N.; Froment, P.; Dupont, J. Adiponectin and resistin: Potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction 2017, 153, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.K.; Otsuka, F.; Shimasaki, S. Role of ERK1/2 in the differential synthesis of progesterone and estradiol by granulosa cells. Biochem. Biophys. Res. Commun. 2001, 289, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Kayampilly, P.P.; Menon, K.M. Inhibition of extracellular signal-regulated protein kinase-2 phosphorylation by dihydrotestosterone reduces follicle-stimulating hormone-mediated cyclin D2 messenger ribonucleic acid expression in rat granulosa cells. Endocrinology 2004, 145, 1786–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosca, L.; Froment, P.; Solnais, P.; Ferré, P.; Foufelle, F.; Dupont, J. Adenosine 5′-monophosphate-activated protein kinase regulates progesterone secretion in rat granulosa cells. Endocrinology 2005, 146, 4500–4513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.Q.; Han, C.S.; Yang, W.; Jin, X.; Hu, Z.Y.; Liu, Y.X. Role of ERK1/2 in FSH induced PCNA expression and steroidogenesis in granulosa cells. Front. Biosci. 2005, 10, 896–904. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.E.; Glister, C.; Lonergan, P.; Martin, F.; Knight, P.G.; Evans, A.C. Functional significance of the signal transduction pathways Akt and Erk in ovarian follicles: In vitro and in vivo studies in cattle and sheep. J. Ovarian. Res. 2008, 1, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayampilly, P.P.; Menon, K.M. Follicle-stimulating hormone inhibits adenosine 5′-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology 2009, 150, 929–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, S.L.; Dasgupta, T.; Cummings, M.; Orsi, N.M. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol. Reprod. Dev. 2014, 81, 284–314. [Google Scholar] [CrossRef] [PubMed]
- Shuang, L.; Jidong, W.; Hongjuan, P.; Zhenwei, Y. Effects of apelin on proliferation and apoptosis in rat ovarian granulosa cells. Clin. Exp. Obstet. Gynecol. 2016, 43, 409–413. [Google Scholar] [PubMed]
- Bertoldo, M.J.; Faure, M.; Dupont, J.; Froment, P. AMPK: A master energy regulator for gonadal function. Front. Neurosci. 2015, 9, 235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandedkar, T.D.; Dharma, S.J. Expression of bcl (xs) and c-myc in atretic follicles of mouse ovary. Reprod. Biomed. Online 2001, 3, 221–225. [Google Scholar] [CrossRef]
- Van Nassauw, L.; Tao, L.; Harrisson, F. Distribution of apoptosis-related proteins in the quail ovary during folliculogenesis: BCL-2, BAX and CPP32. Acta Histochem. 1999, 101, 103–112. [Google Scholar] [CrossRef]
- Kim, J.M.; Yoon, Y.D.; Tsang, B.K. Involvement of the Fas/Fas ligand system in p53-mediated granulosa cell apoptosis during follicular development and atresia. Endocrinology 1999, 140, 2307–2317. [Google Scholar] [CrossRef] [PubMed]
- Tilly, K.I.; Banerjee, S.; Banerjee, P.P.; Tilly, J.L. Expression of the p53 and Wilms’ tumor suppressor genes in the rat ovary: Gonadotropin repression in vivo and immunohistochemical localization of nuclear p53 protein to apoptotic granulosa cells of atretic follicles. Endocrinology 1995, 136, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, W.J.; Hwang, J.Y.; Seol, S.M.; Kim, Y.M.; Lee, Y.L.; Park, J.Y. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3-kinase/Akt pathway. Biochem. Biophys. Res. Commun. 2011, 413, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Gregoraszczuk, E.L.; Rak, A.; Wójtowicz, A.; Ptak, A.; Wojciechowicz, T.; Nowak, K.W. Gh and IGF-I increase leptin receptor expression in prepubertal pig ovaries: The role of leptin in steroid secretion and cell apoptosis. Acta Vet. Hung. 2006, 54, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Benčo, A.; Tandlmajerová, A.; Vašíček, D. Involvement of transcription factor p53 and leptin in control of porcine ovarian granulosa cell functions. Cell Prolif. 2012, 45, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zou, P.; He, Y.; Meng, K.; Quan, F.; Zhang, Y. Effect of luteinizing hormone on goat theca cell apoptosis and steroidogenesis through activation of the PI3K/AKT pathway. Anim. Reprod. Sci. 2018, 190, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.K.; Sellers, W.R. Akt-regulated pathways in prostate cancer. Oncogene 2005, 24, 7465–7474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.H.; Zhang, X. Roles of GRP78 in physiology and cancer. J. Cell Biochem. 2010, 110, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, R.; Ni, M.; Gill, P.; Lee, A.S. Cell surface relocalization of the endoplasmic reticulum chaperone and unfolded protein response regulator GRP78/BiP. J. Biol. Chem. 2010, 285, 15065–15075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Gronow, M.; Selim, M.A.; Papalas, J.; Pizzo, S.V. GRP78: A multifunctional receptor on the cell surface. Antioxid. Redox Signal. 2009, 11, 2299–2306. [Google Scholar] [CrossRef] [PubMed]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar] [PubMed]
- Stoklosowa, S.; Bahr, J.; Gregoraszczuk, E. Some morphological and functional characteristics of cells of the porcine theca interna in tissue culture. Biol. Reprod. 1978, 19, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xu, F.; Pei, H.X.; Zhu, X.; Lin, X.; Song, C.Y.; Liang, Q.H.; Liao, E.Y.; Yuan, L.Q. Vaspin regulates the osteogenic differentiation of MC3T3-E1 through the PI3K-Akt/miR-34c loop. Sci. Rep. 2016, 6, 25578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Park, J.Y.; Kim, J.W.; Yang, S.G.; Jung, J.M.; Kim, M.J.; Park, J.J.; Koo, D.B. Regulation of the Endoplasmic Reticulum Stress by BIP/GRP78 is involved in Meiotic Maturation of Porcine Oocytes In Vitro. Dev. Reprod. 2017, 21, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Manabe, N.; Goto, Y.; Inoue, N.; Nishihara, S.; Miyamoto, H. Expression and activity of Apaf1 and caspase-9 in granulosa cells during follicular atresia in pig ovaries. Reproduction 2003, 126, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Rak-Mardyła, A.; Durak, M.; Gregoraszczuk, E.L. Effects of resistin on porcine ovarian follicle steroidogenesis in prepubertal animals: An in vitro study. Reprod. Biol. Endocrinol. 2013, 11, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Antibody | Host Species | Dilution | Vendor/Product No. |
|---|---|---|---|
| cyclin D | rabbit | 1:1000 | Cell Signalling Technology, USA, product no. 2978S |
| cyclin E | mouse | 1:1000 | Abcam, Cambridge, GB, product no. ab3927 |
| cyclin A | mouse | 1:1000 | Cell Signalling Technology, USA, product no. 4656S |
| caspase-3 | rabbit | 1:1000 | Cell Signalling Technology, USA, product no. 9662S |
| caspase-8 | mouse | 1:500 | ThermoFisher Scientific, USA, product no. MA1-41280 |
| caspase-9 | rabbit | 1:1000 | Invitrogen, Invitrogen, USA, product no. PA5-22252 |
| BCL2 | rabbit | 1:1000 | Cell Signalling Technology, USA, product no. 4223S |
| BAX | rabbit | 1:1000 | Cell Signalling Technology, USA, product no. 2772 |
| p53 | rabbit | 1:1000 | Cell Signalling Technology, USA, product no. 9282S |
| actin | mouse | 1:5000 | Sigma-Aldrich, USA, product no. A5316 |
| anti-rabbit | goat | 1:1000 | Cell Signalling Technology, USA, product no. 7074 |
| anti-mouse | horse | 1:1000 | Cell Signalling Technology, USA, product no. 7076 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Opydo-Chanek, M.; Dupont, J.; Rak, A. In Vitro Effects of Vaspin on Porcine Granulosa Cell Proliferation, Cell Cycle Progression, and Apoptosis by Activation of GRP78 Receptor and Several Kinase Signaling Pathways Including MAP3/1, AKT, and STAT3. Int. J. Mol. Sci. 2019, 20, 5816. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225816
Kurowska P, Mlyczyńska E, Dawid M, Opydo-Chanek M, Dupont J, Rak A. In Vitro Effects of Vaspin on Porcine Granulosa Cell Proliferation, Cell Cycle Progression, and Apoptosis by Activation of GRP78 Receptor and Several Kinase Signaling Pathways Including MAP3/1, AKT, and STAT3. International Journal of Molecular Sciences. 2019; 20(22):5816. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225816
Chicago/Turabian StyleKurowska, Patrycja, Ewa Mlyczyńska, Monika Dawid, Małgorzata Opydo-Chanek, Joelle Dupont, and Agnieszka Rak. 2019. "In Vitro Effects of Vaspin on Porcine Granulosa Cell Proliferation, Cell Cycle Progression, and Apoptosis by Activation of GRP78 Receptor and Several Kinase Signaling Pathways Including MAP3/1, AKT, and STAT3" International Journal of Molecular Sciences 20, no. 22: 5816. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20225816





