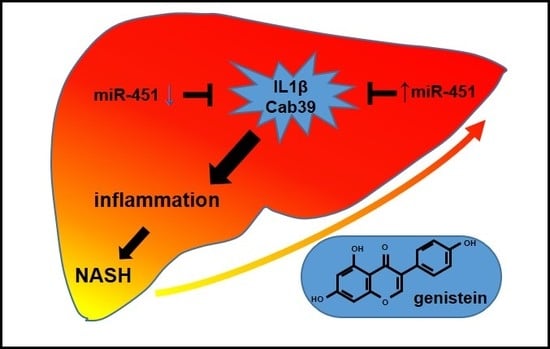
MicroRNA-451 and Genistein Ameliorate Nonalcoholic Steatohepatitis in Mice
Abstract
:1. Introduction
2. Results
2.1. MiR-451 Is Downregulated in Liver Inflammation
2.2. MiR-451 Regulates Inflammation by Targeting IL1β
2.3. Genistein Induced miR-451 Expression
2.4. Genistein Inhibits LPS-Induced Liver Inflammation in Mice
2.5. Genistein Ameliorates NASH in Mice
2.6. The Potential Role of Genistein, miR-451 and Its Target Genes in the Treatment of Liver Cancer
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Animal Model
4.3. Tissue Section, Liver Weight Index and NASH Activity Score
4.4. Colorimetric Evaluation of Liver Tissue
4.5. Liver Homogenate Inflammatory Factors
4.6. Cell Culture and Treatment
4.7. Luciferase Reporter Assay
4.8. Quantitative Real-Time PCR
4.9. Western Blot Analysis
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Exton, J.H.; Lewis, S.B.; Ho, R.J.; Robison, G.A.; Park, C.R. The role of cyclic amp in the interaction of glucagon and insulin in the control of liver metabolism. Ann. N. Y. Acad. Sci. 2010, 185, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zeng, F. Directed hepatic differentiation from embryonic stem cells. Protein Cell 2011, 2, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Luo, Y.; Li, X.; Yi, Y. Differential expression analysis of Golgi apparatus proteomes in hepatocellular carcinomas and the surrounding liver tissues. Hepatol. Res. 2014, 44, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Alatrakchi, N.; Koziel, M. Regulatory T cells and viral liver disease. J. Viral Hepat. 2009, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Bianchi, G.; Tomassetti, S.; Bugianesi, E.; Lenzi, M.; Mccullough, A.J.; Natale, S.; Forlani, G.; Melchionda, N. Nonalcoholic fatty liver disease: A feature of the metabolic syndrome. Diabetes 2001, 50, 1844–1850. [Google Scholar] [CrossRef] [Green Version]
- Mukaiya, M.; Nishi, M.; Miyake, H.; Hirata, K. Chronic liver diseases for the risk of hepatocellular carcinoma: A case-control study in japan. Etiologic association of alcohol consumption, cigarette smoking and the development of chronic liver diseases. Hepato-Gastroenterology 1998, 45, 2328. [Google Scholar]
- Ciernik, I.F. Risk factors for hepatocellular carcinoma in patients with chronic liver diseases. N. Engl. J. Med. 1993, 329, 1897–1898. [Google Scholar]
- Moore, M.S.; Ivanina, E.; Bornschlegel, K.; Qiao, B.; Schymura, M.J.; Laraque, F. Hepatocellular carcinoma and viral hepatitis in New York city. Clin. Infect. Dis. 2016, 63, 1577. [Google Scholar] [CrossRef]
- Li, C.I.; Chen, H.J.; Lai, H.C.; Liu, C.S.; Lin, W.Y.; Li, T.C.; Lin, C.C. Hyperglycemia and chronic liver diseases on risk of hepatocellular carcinoma in Chinese patients with type 2 diabetes–national cohort of taiwan diabetes study. Int. J. Cancer. 2015, 136, 2668–2679. [Google Scholar] [CrossRef]
- Dajani, A.; Abuhammour, A. Treatment of nonalcoholic fatty liver disease: Where do we stand? An overview. Saudi J. Gastroenterol. 2016, 22, 91–105. [Google Scholar]
- Maurice, J.; Manousou, P. Non-alcoholic fatty liver disease. Clin. Med. 2018, 18, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Arrese, M.; Trauner, M. Recent insights into the pathogenesis of nonalcoholic fatty liver disease. Annu. Rev. Pathol. 2018, 13, 321–350. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of free radicals and oxidative stress in nafld/nash. Free Radic. Res. 2013, 47, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Wruck, W.; Graffmann, N.; Kawala, M.; Adjaye, J. Concise review: Current status and future directions on research related to nonalcoholic fatty liver disease. Stem. Cells 2017, 35, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konerman, M.A.; Jones, J.C.; Harrison, S.A. Pharmacotherapy for nash: Current and emerging. J. Hepatol. 2017, 68, 362–375. [Google Scholar] [CrossRef]
- Campo, J.A.D.; Gallego, P.; Grande, L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J. Hepatol. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Mehal, W.Z. The inflammasome in liver injury and non-alcoholic fatty liver disease. Dig. Dis. 2014, 32, 507–515. [Google Scholar] [CrossRef]
- Pin, Y.; Rui, P.; Huimin, P.; Li, Y.; Yan, S.; Li, W.; Tianhui, W.; Ji, Z.; Zheng, Z. Mir-451 suppresses cell proliferation and metastasis in a549 lung cancer cells. Mol. Biotechnol. 2015, 57, 1–11. [Google Scholar]
- Zhang, F.; Huang, W.; Sheng, M.; Liu, T. Mir-451 inhibits cell growth and invasion by targeting cxcl16 and is associated with prognosis of osteosarcoma patients. Tumor Biol. 2015, 36, 2041–2048. [Google Scholar] [CrossRef]
- Hur, W.; Lee, J.H.; Kim, S.W.; Kim, J.H.; Si, H.B.; Kim, M.; Hwang, D.; Kim, Y.S.; Park, T.; Um, S.J. Downregulation of microrna-451 in non-alcoholic steatohepatitis inhibits fatty acid-induced proinflammatory cytokine production through the ampk/akt pathway. Int. J. Biochem. Cell Biol. 2015, 64, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Li, H.P.; Zeng, X.C.; Zhang, B.; Long, J.T.; Zhou, B.; Tan, G.S.; Zeng, W.X.; Chen, W.; Yang, J.Y. Mir-451 inhibits cell proliferation in human hepatocellular carcinoma through direct suppression of IKK-β. Carcinogenesis 2013, 34, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic activity of genistein aglycone in postmenopausal women: Evidence from a post-hoc analysis of a multicenter randomized controlled trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef] [Green Version]
- Gan, M.; Zheng, T.; Shen, L.; Tan, Y.; Fan, Y.; Shuai, S.; Bai, L.; Li, X.; Wang, J.; Zhang, S.; et al. Genistein reverses isoproterenol-induced cardiac hypertrophy by regulating mir-451/timp2. Biomed. Pharm. 2019, 112, 108618. [Google Scholar] [CrossRef]
- Pan, X.; Wang, R.; Wang, Z.X. The potential role of mir-451 in cancer diagnosis, prognosis, and therapy. Mol. Cancer Ther. 2013, 12, 1153–1162. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Yoshitomi, H.; Furu, M.; Ishikawa, M.; Shibuya, H.; Ito, H.; Matsuda, S. Microrna-451 down-regulates neutrophil chemotaxis via p38 mapk. Arthritis Rheumatol. 2014, 66, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Zeng, N.; Huang, R.; Li, N.; Jiang, H.; Li, R.; Wang, F.; Chen, W.; Xia, M.; Wang, Q. Mir-451a attenuates free fatty acids–mediated hepatocyte steatosis by targeting the thyroid hormone responsive spot 14 gene. Mol. Cell. Endocrinol. 2018, 474, 260–271. [Google Scholar] [CrossRef]
- Kudo, H.; Takahara, T.; Yata, Y.; Kawai, K.; Zhang, W.; Sugiyama, T. Lipopolysaccharide triggered TNF-α-induced hepatocyte apoptosis in a murine non-alcoholic steatohepatitis model. J. Hepatol. 2009, 51, 168–175. [Google Scholar] [CrossRef]
- Leverence, J.T.; Medhora, M.; Konduri, G.G.; Sampath, V. Lipopolysaccharide-induced cytokine expression in alveolar epithelial cells: Role of pkcζ-mediated p47phox phosphorylation. Chem.-Biol. Interact. 2011, 189, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sugita, H.; Kaneki, M.; Tokunaga, E.; Sugita, M.; Koike, C.; Yasuhara, S.; Tompkins, R.G.; Martyn, J.A.J. Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonicelli, F.; Brown, D.; Parmentier, M.; Drost, E.M.; Hirani, N.; Rahman, I.; Donaldson, K.; Macnee, W. Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant nacystelyn. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.D.; Ha, B.J. The effects of paeoniflorin on LPS-induced liver inflammatory reactions. Arch. Pharmacal Res. 2010, 33, 959–966. [Google Scholar] [CrossRef]
- Gäbele, E.; Dostert, K.; Hofmann, C.; Wiest, R.; Schölmerich, J.; Hellerbrand, C.; Obermeier, F. Dss induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J. Hepatol. 2011, 55, 1181–1183. [Google Scholar] [CrossRef]
- Abbott, A.L.; Alvarez-Saavedra, E.; Miska, E.A.; Lau, N.C.; Bartel, D.P.; Horvitz, H.R.; Ambros, V. The let-7 microrna family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in caenorhabditis elegans. Dev. Cell 2006, 10, 271. [Google Scholar] [CrossRef] [Green Version]
- Stark, A.; Brennecke, J.; Russell, R.B.; Cohen, S.M. Identification of drosophila microrna targets. PLoS Biol. 2003, 1, E60. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, L.; Zhang, Y.; Li, P.; Wu, Y.; Lin, Y. Mir-26b-3p regulates osteoblast differentiation via targeting estrogen receptor α. Genomics 2018, 111, 1089–1096. [Google Scholar] [CrossRef]
- Ørom, U.A.; Nielsen, F.C.; Lund, A.H. Microrna-10a binds the 5′utr of ribosomal protein mrnas and enhances their translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef]
- Nemetz, A.; Nosti-Escanilla, M.P.; Molnár, T.; Köpe, A.; Kovács, A.; Fehér, J.; Tulassay, Z.; Nagy, F.; García-González, M.A.; Peña, A.S. Il1b gene polymorphisms influence the course and severity of inflammatory bowel disease. Immunogenetics 1999, 49, 527–531. [Google Scholar] [CrossRef]
- Mirea, A.M.; Tack, C.J.; Chavakis, T.; Joosten, L.A.B.; Toonen, E.J.M. IL-1 family cytokine pathways underlying nafld: Towards new treatment strategies. Trends Mol. Med. 2018, 24, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.E.; Handa, P.; Aouizerat, B.; Wilson, L.; Vemulakonda, L.A.; Yeh, M.M.; Kowdley, K.V.; Network, N.C. Increased parenchymal damage and steatohepatitis in caucasian non-alcoholic fatty liver disease patients with common il1b and il6 polymorphisms. Aliment. Pharm. Ther. 2016, 44, 1253–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godlewski, J.; Nowicki, M.O.; Bronisz, A.; Nuovo, G.; Palatini, J.; De Lay, M.; Van Brocklyn, J.; Ostrowski, M.C.; Chiocca, E.A.; Lawler, S.E. Microrna-451 regulates lkb1/ampk signaling and allows adaptation to metabolic stress in glioma cells. Mol. Cell 2010, 37, 620–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula, M.L.; Rodrigues, D.H.; Teixeira, H.C.; Barsante, M.M.; Souza, M.A.; Ferreira, A.P. Genistein down-modulates pro-inflammatory cytokines and reverses clinical signs of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2008, 8, 1291–1297. [Google Scholar] [CrossRef]
- Yin, Y.M.; Liu, H.H.; Zheng, Z.C.; Lu, R.R.; Jiang, Z.Q. Genistein can ameliorate hepatic inflammatory reaction in nonalcoholic steatohepatitis rats. Biomed. Pharm. 2019, 111, 1290–1296. [Google Scholar]
- Zhong, H.; Liu, H.; Jiang, Z. Genistein ameliorates fat accumulation through ampk activation in fatty acid-induced brl cells. J. Food Sci. 2017, 82, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Amanat, S.; Eftekhari, M.H.; Fararouei, M.; Bagheri Lankarani, K.; Massoumi, S.J. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 2018, 37, 1210–1215. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Li, H.; Huang, L.; Sun, Q.; Dong, Y.; Tian, C.; Gao, S.; Dong, H.; Guan, D.; et al. Identification and characterization of micrornas in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010, 20, 1128–1137. [Google Scholar] [CrossRef]
- Nothnick, W.B.; Healy, C. Estrogen induces distinct patterns of microrna expression within the mouse uterus. Reprod. Sci. 2010, 17, 987–994. [Google Scholar] [CrossRef]
- Harrison, R.M.; Phillippi, P.P.; Swan, K.F.; Henson, M.C. Effect of genistein on steroid hormone production in the pregnant rhesus monkey. Proc. Soc. Exp. Biol. Med. 2010, 222, 78–84. [Google Scholar]
- Murakami, Y.; Abe, T.; Hashiguchi, A.; Yamaguchi, M.; Saito, A.; Sakamoto, M. Color correction for automatic fibrosis quantification in liver biopsy specimens. J. Pathol. Inform. 2013, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Dagan, A.; Roan, P.; Rosen, J.; Sinanan, M.; Gupta, M.; Hannaford, B. CIELab and sRGB color values of in vivo normal and grasped porcine liver. Stud. Health Technol. Inform. 2007, 125, 109–111. [Google Scholar] [PubMed]
- Watanabe, S.; Hashimoto, E.; Ikejima, K.; Uto, H.; Ono, M.; Sumida, Y.; Seike, M.; Takei, Y.; Takehara, T.; Tokushige, K.; et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J. Gastroenterol. 2015, 50, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Kwon, S.W.; Lee, Y.H.; Kaya, P.; Kim, J.M.; Ahn, C.; Jung, E.-M.; Lee, G.-S.; An, B.-S.; Jeung, E.-B.; et al. Dietary intake of genistein suppresses hepatocellular carcinoma through ampk-mediated apoptosis and anti-inflammation. BMC Cancer 2019, 19, 6. [Google Scholar] [CrossRef]
- Fumiaki, S.; Etsuro, H.; Koji, K.; Akira, M.; Takeshi, F.; Satoko, T.; Soken, T.; Gozoh, T.; Shinji, U.; Kazuharu, S. Microrna profile predicts recurrence after resection in patients with hepatocellular carcinoma within the milan criteria. PLoS ONE 2011, 6, e16435. [Google Scholar]
- Zhang, Q.; Wang, H.; Mao, C.; Sun, M.; Dominah, G.; Chen, L.; Zhuang, Z. Fatty acid oxidation contributes to il-1β secretion in m2 macrophages and promotes macrophage-mediated tumor cell migration. Mol. Immunol. 2018, 94, 27–35. [Google Scholar] [CrossRef]
- Liu, H.; Liao, R.; He, K.; Zhu, X.; Li, P.; Gong, J. The smac mimetic birinapant attenuates lipopolysaccharide-induced liver injury by inhibiting the tumor necrosis factor receptor–associated factor 3 degradation in kupffer cells. Immunol. Lett. 2017, 185, 79–83. [Google Scholar] [CrossRef]
- Gan, M.; Yang, D.; Fan, Y.; Du, J.; Shen, L.; Li, Q.; Jiang, Y.; Tang, G.; Li, M.; Wang, J.; et al. Bidirectional regulation of genistein on the proliferation and differentiation of c2c12 myoblasts. Xenobiotica 2017, 1–15. [Google Scholar] [CrossRef]
- Gan, M.; Du, J.; Shen, L.; Yang, D.; Jiang, A.; Li, Q.; Jiang, Y.; Tang, G.; Li, M.; Wang, J. Mir-152 regulates the proliferation and differentiation of c2c12 myoblasts by targeting e2f3. In Vitro Cell. Dev. Biol.–Anim. 2018, 54, 304–310. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shen, L.; Gan, M.; Li, Q.; Wang, J.; Li, X.; Zhang, S.; Zhu, L. Microrna-200b regulates preadipocyte proliferation and differentiation by targeting klf4. Biomed. Pharm. 2018, 103, 1538. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, M.; Shen, L.; Fan, Y.; Tan, Y.; Zheng, T.; Tang, G.; Niu, L.; Zhao, Y.; Chen, L.; Jiang, D.; et al. MicroRNA-451 and Genistein Ameliorate Nonalcoholic Steatohepatitis in Mice. Int. J. Mol. Sci. 2019, 20, 6084. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20236084
Gan M, Shen L, Fan Y, Tan Y, Zheng T, Tang G, Niu L, Zhao Y, Chen L, Jiang D, et al. MicroRNA-451 and Genistein Ameliorate Nonalcoholic Steatohepatitis in Mice. International Journal of Molecular Sciences. 2019; 20(23):6084. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20236084
Chicago/Turabian StyleGan, Mailin, Linyuan Shen, Yuan Fan, Ya Tan, Ting Zheng, Guoqing Tang, Lili Niu, Ye Zhao, Lei Chen, Dongmei Jiang, and et al. 2019. "MicroRNA-451 and Genistein Ameliorate Nonalcoholic Steatohepatitis in Mice" International Journal of Molecular Sciences 20, no. 23: 6084. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20236084