Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine
Abstract
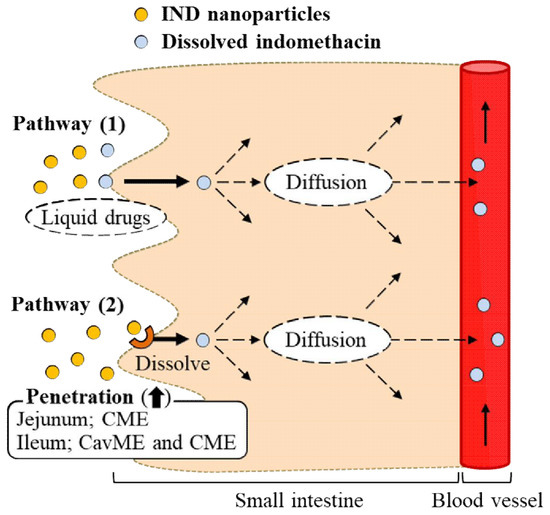
:1. Introduction
2. Results
2.1. Design of Oral Formulation Containing Indomethacin Nanoparticles
2.2. Stability of the Oral Formulation Containing Indomethacin Nanoparticles
2.3. Effect of the Energy-Dependent Endocytosis on the Transintestinal Penetration of Indomethacin Nanoparticles Using Caco-2 Cell Monolayers
2.4. Effect of Energy-Dependent Endocytosis on the Transintestinal Penetration of Indomethacin Nanoparticles in the Rat Jejunum and Ileum
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Preparation of IND-NPs
4.4. Analysis of Particle Size and Number of Indomethacin Nanoparticles
4.5. Evaluation of Dispersibility in IND-NPs
4.6. Measurement of Indomethacin Penetration through Caco-2 Cell Monolayers
4.7. Measurement of Indomethacin Penetration through Removed Small Intestine in Rats
4.8. Inhibition of Energy-Dependent Endocytosis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | one-way analysis of variance |
| AFM | atomic force microscope |
| AUC | area under the drug concentration-time curve |
| BA | bioavailability |
| Caco-2 | human epithelial colorectal adenocarcinoma cell line |
| CavME | caveolae-dependent endocytosis |
| CME | clathrin-dependent endocytosis |
| COX | cyclooxygenase |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| DDS | drug delivery systems |
| IND-MPs | oral formulation containing indomethacin microparticles |
| IND-NPs | oral formulation containing indomethacin nanoparticles |
| IND-solution | liquid indomethacin |
| MP | macropinocytosis |
| MC | methylcellulose |
| NO | nitric oxide |
| NSAID | non-steroidal anti-inflammatory drug |
| PG | prostaglandin |
| S.D. | standard deviation |
| S.E. | standard error |
| TER | transepithelial electrical resistance |
References
- Graham, D.Y.; Opekun, A.R.; Willingham, F.F.; Qureshi, W.A. Visible small-intestinal mucosal injury in chronic NSAID users. Clin. Gastroenterol. Hepatol. 2005, 3, 55–59. [Google Scholar] [CrossRef]
- Higuchi, K.; Umegaki, E.; Watanabe, T.; Yoda, Y.; Morita, E.; Murano, M.; Tokioka, S.; Arakawa, T. Present status and strategy of NSAIDs-induced small bowel injury. J. Gastroenterol. 2009, 44, 879–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, M.; Kawahara, R.; Hashimura, H.; Yamanaka, N.; Iimori, M.; Amagase, K.; Kato, S.; Takeuchi, K. Dopamine D(2)-receptor antagonists ameliorate indomethacin-induced small intestinal ulceration in mice by activating alpha7 nicotinic acetylcholine receptors. J. Pharmacol. Sci. 2011, 116, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Yasuda, M.; Hashimura, H.; Amagase, K.; Kato, S.; Takeuchi, K. Activation of alpha7 nicotinic acetylcholine receptors ameliorates indomethacin-induced small intestinal ulceration in mice. Eur. J. Pharmacol. 2011, 650, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, Y.; Fujino, H.; Otake, S.; Murayama, T. Indomethacin antagonizes EP(2) prostanoid receptor activation in LS174T human colon cancer cells. Eur. J. Pharmacol. 2012, 680, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, T.; Tanaka, A.; Miyazawa, T.; Kato, S.; Takeuhi, K. 16,16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig. Dis. Sci. 2002, 47, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, R.; Ohno, R.; Tanigami, M.; Takeuchi, K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J. Pharmacol. Exp. Ther. 2006, 318, 691–699. [Google Scholar] [CrossRef]
- Boelsterli, U.A.; Redinbo, M.R.; Saitta, K.S. Multiple NSAID-induced hits injure the small intestine: Underlying mechanisms and novel strategies. Toxicol. Sci. 2013, 131, 654–667. [Google Scholar] [CrossRef]
- Takeuchi, K.; Tanaka, A.; Kato, S.; Amagase, K.; Satoh, H. Roles of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. Clin. Chim. Acta 2010, 411, 459–466. [Google Scholar] [CrossRef]
- Boltze, K.; Brendler, O.; Jacobi, H.; Optiz, W.; Raddatz, S.; Seidel, P.R.; Vollbrecht, D. Chemical structure and anti-inflammatory activity in the group of substituted indole-3 acetic acids. Arzneim-Forsch/Drug Res. 1980, 30, 1314–1325. [Google Scholar]
- Jacobi, H.; Dell, H.D. On the pharmacodynamics of acemetacin. Arzneim-Forsch/Drug Res. 1980, 30, 1348–1362. [Google Scholar]
- Kumakura, S.; Mishima, M.; Kobayashi, S.; Abe, S.; Yamada, K.; Tsurufuji, S. Inhibitory effect of indomethacin farnesyl, a novel anti-inflammatory prodrug, on carrageenan-induced inflammation in rats. Agents Actions 1990, 29, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Singh, S.R.; Yilma, A.N.; Agee, R.D., 2nd; Taha, M.; Dennis, V.A. Poly(lactic acid)-poly(ethylene glycol) nanoparticles provide sustained delivery of a Chlamydia trachomatis recombinant MOMP peptide and potentiate systemic adaptive immune responses in mice. Nanomedicine 2014, 10, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Kalkanidis, M.; Pietersz, G.A.; Xiang, S.D.; Mottram, P.L.; Crimeen-Irwin, B.; Ardipradja, K.; Plebanski, M. Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods 2006, 40, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. Effect of solid nanoparticle of indomethacin on therapy for rheumatoid arthritis in adjuvant-induced arthritis rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Gomez-Orellana, I. Challenges for the Oral Delivery of Macromolecules. Nat. Rev. Drug Discovery 2003, 2, 289–295. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier Properties of Mucus. Adv. Drug Delivery Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Rappoport, J.Z. Focusing on clathrin-mediated endocytosis. Biochem. J. 2008, 412, 415–423. [Google Scholar] [CrossRef]
- Wang, J.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. More effective nanomedicines through particle design. Small 2011, 7, 1919–1931. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Delivery Rev. 2017, 108, 51–67. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagai, N.; Yoshioka, C.; Ito, Y. Topical therapies for rheumatoid arthritis by gel ointments containing indomethacin nanoparticles in adjuvant-induced arthritis rat. J. Oleo Sci. 2015, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and antiinflammatory effect of a novel gel system containing ketoprofen solid nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int. J. Nanomed. 2018, 13, 5215–5229. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. Int. J. Mol. Sci. 2015, 16, 29329–29344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Baracia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef] [PubMed]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef] [Green Version]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčĭř, T.; Kverka, M.; Zákostelská, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2008, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, D.; Qin, M.; Chen, B.; Song, S.; Dai, W.; Zhang, H.; Wang, X.; Wang, Y.; He, B.; et al. Intestinal Mucin Induces More Endocytosis but Less Transcytosis of Nanoparticles across Enterocytes by Triggering Nanoclustering and Strengthening the Retrograde Pathway. ACS. Appl. Mater Interfaces. 2018, 10, 11443–11456. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]







| Treatment | Jc (pmol/cm2/h) | Kp (×10−5/h) | Km (×10−3) | τ (h) | D (×10−3 cm2/h) |
|---|---|---|---|---|---|
| Normal (37 °C treatment) | 2.6 ± 0.6 | 5.0 ± 1.1 | 1.6 ± 0.4 | 0.69 ± 0.12 | 2.7 ± 0.6 |
| 4 °C treatment | 0.2 ± 0.1*,# | 0.3 ± 0.1*,# | 0.2 ± 0.1*,# | 1.16 ± 0.18*,# | 1.4 ± 0.3*,# |
| Vehicle | 3.9 ± 1.0 | 7.5 ± 1.5 | 2.6 ± 0.3 | 0.72 ± 0.11 | 2.5 ± 0.4 |
| Nystatin | 3.6 ± 0.9 | 6.9 ± 1.3 | 2.4 ± 0.3 | 0.72 ± 0.10 | 2.6 ± 0.4 |
| Dynasore | 1.9 ± 0.1*,# | 3.9 ± 0.4*,# | 1.9 ± 0.6*,# | 0.85 ± 0.15 | 2.0 ± 0.7 |
| Rottlerin | 3.8 ± 1.0 | 7.1 ± 1.1 | 2.5 ± 0.4 | 0.70 ± 0.13 | 2.3 ± 0.4 |
| Cytochalasin D | 3.9 ± 1.2 | 7.3 ± 1.3 | 2.6 ± 0.5 | 0.71 ± 0.11 | 2.3 ± 0.4 |
| Nys-Dyn | 0.7 ± 0.2*,# | 1.2 ± 0.4*,# | 0.5 ± 0.1*,# | 0.71 ± 0.27 | 2.3 ± 0.7 |
| Treatment | Jc (pmol/cm2/h) | Kp (×10−4/h) | Km (×10−3) | τ (h) | D (×10−3 cm2/h) |
|---|---|---|---|---|---|
| Normal (37 °C treatment) | 7.4 ± 1.1 | 1.3 ± 0.2 | 8.9 ± 1.3 | 1.21 ± 0.14 | 1.4 ± 0.1 |
| 4 °C treatment | – | – | – | – | – |
| Vehicle | 7.5 ± 1.0 | 1.4 ± 0.3 | 9.0 ± 0.9 | 1.18 ± 0.11 | 1.5 ± 0.2 |
| Nystatin | 5.0 ± 1.4*,# | 0.9 ± 0.2*,# | 8.1 ± 1.8 | 1.65 ± 0.37 | 1.3 ± 0.4 |
| Dynasore | 2.4 ± 0.1*,# | 0.5 ± 0.1*,# | 2.6 ± 0.7*,# | 0.95 ± 0.21 | 1.9 ± 0.4 |
| Rottlerin | 6.8 ± 0.7 | 1.0 ± 0.2 | 8.7 ± 1.0 | 1.09 ± 0.11 | 1.5 ± 0.4 |
| Cytochalasin D | 7.7 ± 1.4 | 1.4 ± 0.3 | 9.1 ± 0.9 | 1.14 ± 0.13 | 1.5 ± 0.3 |
| Nys-Dyn | 1.4 ± 0.4*,# | 0.3 ± 0.1*,# | 1.9 ± 0.4*,# | 0.92 ± 0.20 | 1.8 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030476
Ishii M, Fukuoka Y, Deguchi S, Otake H, Tanino T, Nagai N. Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. International Journal of Molecular Sciences. 2019; 20(3):476. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030476
Chicago/Turabian StyleIshii, Miyu, Yuya Fukuoka, Saori Deguchi, Hiroko Otake, Tadatoshi Tanino, and Noriaki Nagai. 2019. "Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine" International Journal of Molecular Sciences 20, no. 3: 476. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms20030476